How to have Polygenically Screened Children
Polygenic screening is a method for modifying the traits of future children via embryo selection. If that sounds like gobbledygook, then think of it a bit like choosing stats for your baby.
That may sound amazing. It may sound like science fiction. It may even sound horribly dystopian. But whatever your feelings, it is in fact possible. And these benefits are available right now for a price that, while expensive, is within reach for most middle-class families.
On a more serious note, there is limited selection power available with today’s technologies, so you will not be able to have a baby Einstein unless you are already a Nobel laureate. But polygenic screening will allow you to decrease your child’s risk of common diseases by 10-60%, reduce their risk of mental disorders, and increase their IQ by somewhere between 3 and 8 points. If you are willing to wait a few years, you may be able to increase IQ by up to 13 points. Including the cost of IVF and testing, these benefits are available for between $30k-100k depending on the mother’s age, how strong of a benefit you want and what kinds of traits you want to select for.
There has been quite a bit of discussion of this topic on LessWrong and adjacent communities but very little concrete advice for would-be parents who are curious whether the benefits are worth the price, particularly for those who have no other reason to do IVF. The purpose of this post is to fill that gap by addressing costs, potential medical complications, choice of clinic, which labs are best, and how age and infertility diagnosis affect the expected benefits.
This is a long post and I expect most people will not want to read the whole thing. If this is you, please use the section selector in the sidebar to navigate to the section you are most interested in. You may want to simply skip to the section titled “The Benefits of Polygenic Embryo Screening”.
Background on IVF
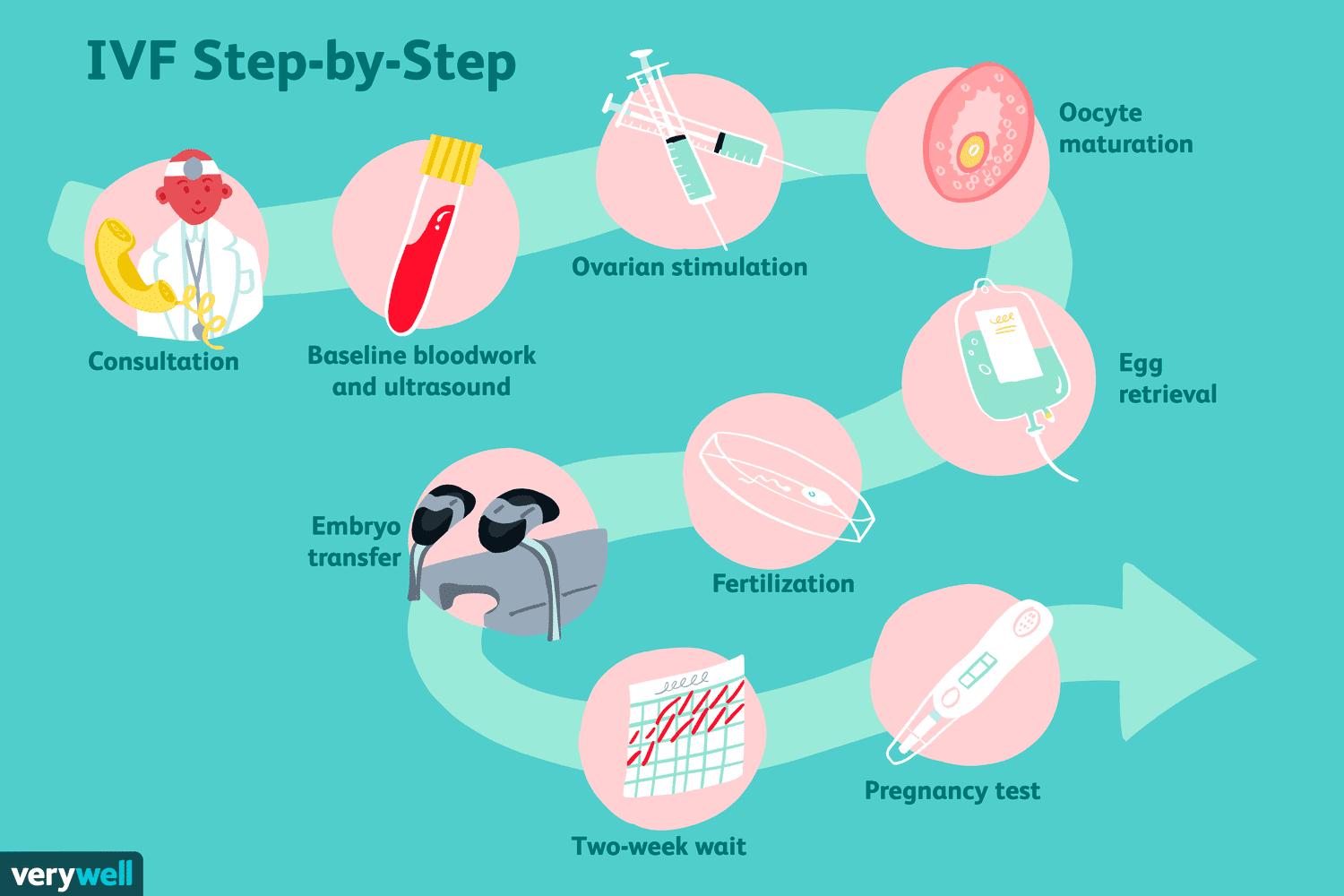
Wait, what even is polygenic embryo selection?
Embryo selection is all about picking an embryo to (hopefully) turn into a baby. This occurs during the process of In-Vitro Fertilization, or IVF. In the typical IVF cycle, a couple goes into a fertility clinic because they want to have a baby. Usually this is because they’ve been having trouble conceiving naturally, but couples also seek out IVF when they want to do genetic testing, select the sex of their child, or to preserve fertility for later pregnancy.
The doctor conducts a bunch of medical tests, and if they all check out, the woman begins a hormone regimen that will stimulate an abnormally large number of her eggs to mature all at once.
At the end of the regiment, the doctor extracts a bunch of mature eggs from the woman’s ovaries, which are then fertilized using the father’s sperm and grown in a lab dish for 4-7 days. When the embryo has finished growing, there are often four or more that can be implanted in the mother. Most couples do not want four children, so a choice must be made about which embryo to pick.
In ye olden days, doctors would often just transfer all the embryos at once in the hope that at least one of them would result in a baby. Sometimes this would work well; one of the embryos would happen to stick and the parents would be very happy. Other times it would work a little too well and more than one of the embryos would implant. This is why twin births are so much more common during IVF than during normal pregnancy.
Transferring multiple embryos at a time is less common nowadays because IVF clinics have figured out how to reduce the odds of failed pregnancy using genetic testing. With a higher chance of live birth from a single embryo transfer, the risk of a failed embryo transfer is outweighed by the risks of a twin pregnancy. The outcomes for twin births are on average worse than for single pregnancies. Twins are more likely to be born preterm, develop health problems, and put excess stress on the mother’s body.
This brings us back to embryo selection; the doctor or embryologist has to make a choice about which embryo to transfer first. All clinics have to make this choice, so all practice embryo selection of some kind. But the criteria for selecting the embryo have, until recently, been pretty dumb.
The standard practice is for an embryologist to look at all the embryos under a microscope and pick the one that looks the prettiest. I am not kidding. The embryologist will rank the embryos from best to worst based on their “morphology”, which accounts for factors like their rotational symmetry and whether or not they have a dark and rough colored appearance.
To be fair to the embryologists, this method is better than just randomly picking an embryo; embryos with particularly bad morphology gradings do actually have a lower chance of resulting in a live birth. And for a long time, there was simply no other option But times have changed and we can now select embryos by DNA rather than simply the appearance of their cells under a microscope.
But how do they even get an embryo’s DNA?
All the best techniques for genotyping rely on destructive sequencing, meaning the cells whose DNA is read must be destroyed. Embryos don’t have very many cells. So how do we get information about what’s in its genome without destroying it?
It turns out that after roughly five days of development, embryos possess a very interesting property; one may remove up to about 10 cells with little to no measurable impact on the embryo’s ability to develop into a healthy child. The embryo can regenerate up to about 10% of its mass! That’s the equivalent of losing and then regrowing both your arms as an adult. This is very fortunate for us because these cells contain a treasure trove of information.
The most common thing IVF clinics look for is aneuploidy, which is a medical term meaning “this embryo has an abnormal number of chromosomes”. The term for this type of testing is “PGT-A”, and it’s performed in roughly half of all IVF cycles in the US today.
Human embryos with the wrong number of chromosomes are surprisingly common, both among IVF patients and natural pregnancy. But this wasn’t very well understood before the first use of pre-implantation genetic testing in the late 1980s.
IVF Doctors started wondering why so many transfers were failing to result in pregnancy, or resulting in pregnancy followed by very early miscarriage. They discovered that roughly a third(!) of all pregnancies, both natural and via IVF have chromosomal abnormalities. Most of the time these go undetected because their immediate effect is to result in the arrest of the embryo’s growth, or to cause a very early miscarriage (often before the woman even knows she is pregnant).
IVF clinics began testing embryos for aneuploidy in the early 1980s. But in the late 2000s, something happened that completely changed the landscape of genetic testing.
Why Polygenic Screening wasn’t possible before 2015
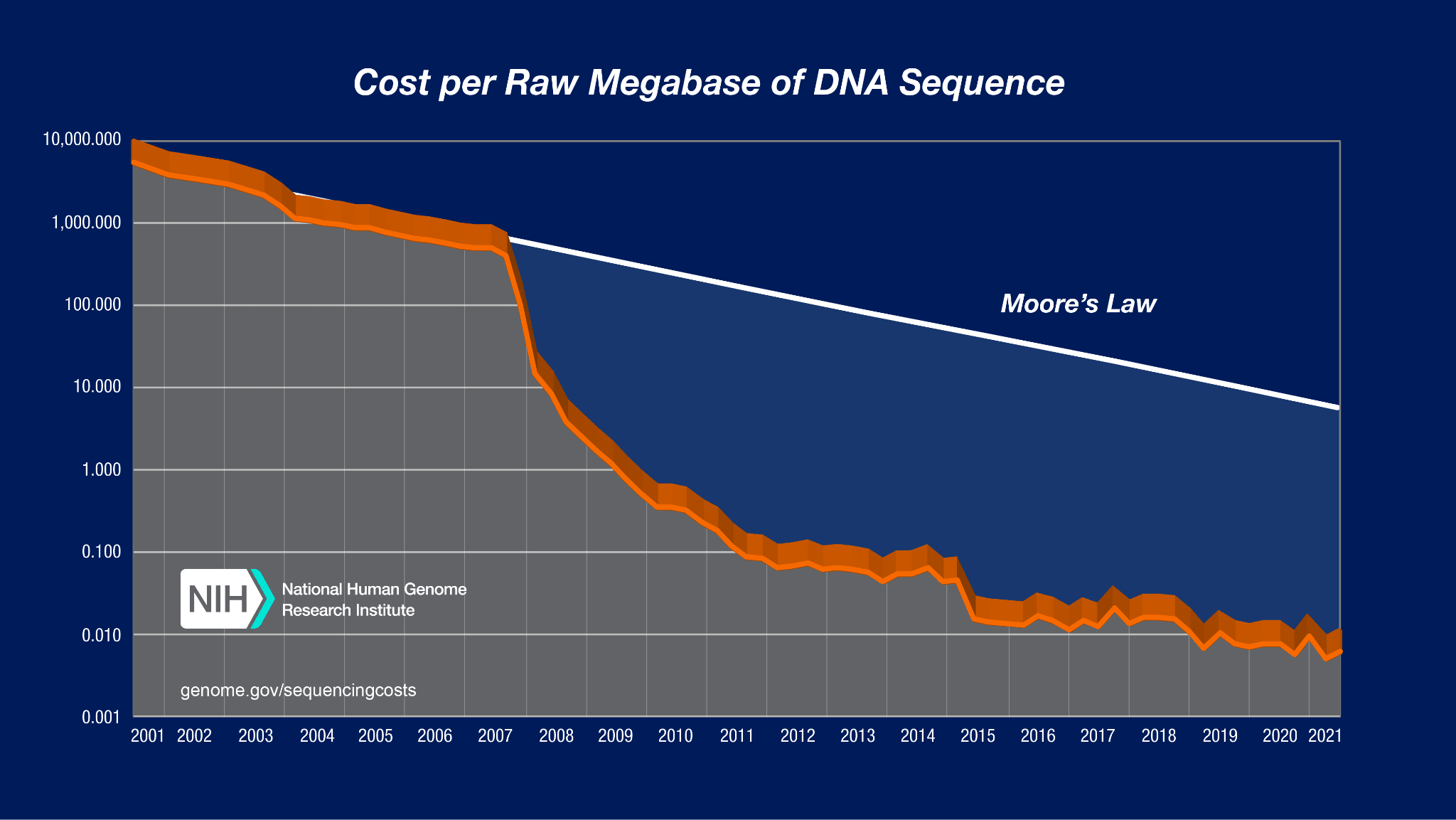
The cost of sequencing a fixed amount of DNA has declined dramatically since Fred Sanger and his team pioneered the first methods in 1977. There is a kind of “Moore’s law of sequencing” in which the cost of sequencing a fixed amount of DNA has declined exponentially over time.
However, something incredibly dramatic happened to DNA sequencing in about 2007. Take a look at this graph:
I don’t think I’ve ever seen a graph that looks like this anywhere else. Between 2007 and 2010, the cost of sequencing a megabase of DNA dropped by a factor of a million! That unbelievable, super exponential drop was made possible by a technology called “Next Generation Sequencing”.
By the mid-2010s, you could genotype all the parts of a person’s DNA most likely to differ from other people’s for under $100. At that price point, it became possible to gather genomes from hundreds of thousands of people and assemble them into giant databases that researchers could access.
This was incredibly important, because you NEED hundreds of thousands of samples to make good genetic predictors. It turns out that most of the traits we care about like heart disease risk or intelligence or attractiveness are determined not by a handful of high-impact genes, but by the cumulative effect of thousands of genes, each of which has only a tiny impact.
Take educational attainment. Educational attainment is not the most heritable trait, but because research on it is more politically acceptable in a university environment than the direct study of intelligence, we know quite a bit about its genetic roots. The latest large-scale study of it included data from 2.7 million participants. Among all genes identified, the one with the single largest effect size only increased the amount of time you spent in school by at most 2.8 weeks (see section 3.4). That’s it! The average gene has a tiny, tiny impact on how long you spend in school. The predictor used 2,925 genes to explain just 15% of the variance in how many years of school a person completed.
So you actually NEED these gigantic databases to explain more than a tiny fraction of the variance in complex traits. This is why polygenic embryo selection was impossible before about 2016; there just wasn’t enough data to figure out which genes did what.
How do they know which genes do what?
Once you have a giant biobank and information about people’s traits and diseases, you still need to figure out which genes do what. I mentioned an educational attainment predictor in the section above, but I didn’t explain how they created it. So how did they do it?
The answer is actually not too complicated: a researcher will use one of these gigantic biobanks plus a machine learning model to identify which genetic variants are associated with an increase or decrease in a given trait.
The dumbest possible way to do this is with a Genome Wide Association Study, or GWAS. It works something like this:
Let’s say there’s a gene with two different variants commonly present in the biobank population. 96% of participants have an “A” at some particular location in the gene, but 4% have a “T” instead. We want to know whether having a “T” makes you taller.
A GWAS just measures the average height of people with an A and the height of people with a T to see if they’re different. Then it uses a statistical significance test to see if the result could have plausibly been the result of random chance. If not, the researchers reason that the gene is having an effect on height. If it passes this test, it is added to the “list of important genes for height”.
For such a dumb method, this works remarkably well. Height predictors created using GWAS results correlate with actual height at about 0.55.
The smarter way to do this is to use some kind of machine learning method like LASSO. This will give you a better predictor for the same amount of data. But for some reason I still don’t really understand, academia almost exclusively uses GWAS.
Correlation or causation?
“OK”, you might say. “That’s well and good, but how do we know that these genetic differences are actually CAUSING someone to be taller or smarter rather than just spuriously correlated with height?”
The main reason this is possible is because nature has already conducted a randomized control trial on our behalf. Every time your body produces a sperm or egg cell, your DNA is more or less randomly mixed up and half of it is given to the reproductive cell. This means that, conditional on parental genomes, sibling genomes are randomized!
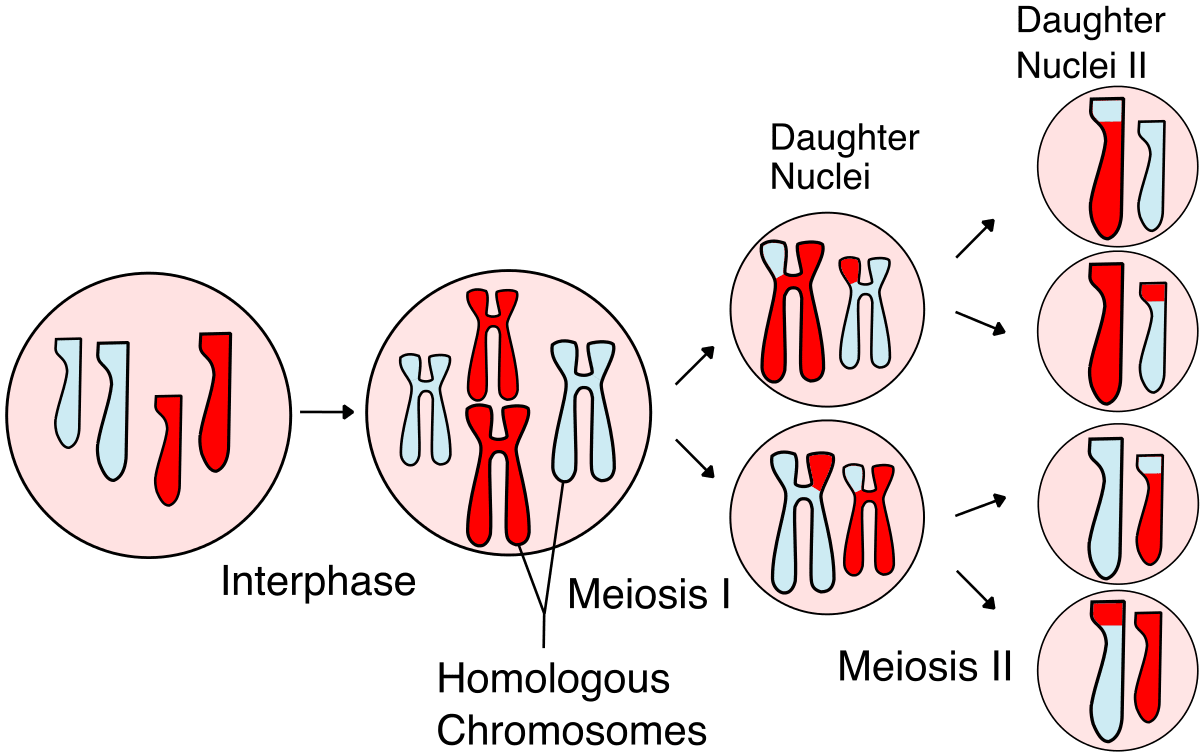
In turn, this means that if a gene can predict differences between siblings, you can be quite confident that it is in fact CAUSING the difference. This is actually quite a remarkable fact, and one that underpins the entire reason for believing embryo selection should work.
There is one asterisk here; though a sibling GWAS can tell you where the causal variant is, it usually can only narrow down the list of candidates to perhaps 10 distinct variants within a region of very roughly 100,000 base pairs. This is sufficient for embryo selection because that set of 10 base pairs will almost always be inherited together. But if sometime down the line we want to do embryo editing, it will require us to either pinpoint the causal variant precisely or to edit all 10 variants that have a decent chance of causing the observed change.
Another crucial insight from these studies is that nearly all of the genetic differences between humans can be explained by additive effects; there are very few gene-gene interactions going on; If gene A makes you taller, it doesn’t depend on gene B being present to work its magic. It’s a strong, independent gene that don’t need no help.
This fact is extremely important because it makes both evolution and embryo selection possible. There is a common misconception that genes are tied together in a hopelessly complex web and that if we mess with one part of it the whole thing will come crashing down. While that may be true for genes that are universally present in the human population, it is very rarely true for genes that commonly vary between people.
You have the predictors. Now what?
Once you have created genetic predictors using GWAS or LASSO or some other method, you can then feed in the embryo’s DNA to the trained model and get a prediction of each embryo’s expected trait value. You do this for every predictor you have (or at least those you care about), and then pick an embryo based on the results.
But there’s one last question to answer: which traits should you care about? If one embryo has a 20% chance of getting breast cancer and a 10% chance of getting heart disease, is that better or worse than an embryo with a 10% risk of breast cancer and a 20% risk of heart disease? Or how about one that has a high risk of both but is also predicted to have an IQ 5 points above average?
There is no universally agreed-upon method for making the choice about which embryo to implant. My personal hope is that someone (maybe even me!) makes a tool to assess what parents find important and then ranks embryos according to those criteria.
The Benefits of Polygenic Embryo Screening
| Category | Trait | Improvement Range | Publicly Available? |
|---|---|---|---|
| Non-disease | Intelligence | +1.6-7.5 IQ points | No* |
| Non-disease | Height | 1-6 cm | No* |
| Non-disease | Personality | ¯\_(ツ)_/¯ | No* |
| Disease | Alzheimer’s | 15-50% reduction | Yes |
| Disease | Atrial Fibrillation | 10-50% | Yes |
| Disease | Asthma | 3-50% | Yes |
| Disease | Breast Cancer | 3-50% | Yes |
| Disease | Basal Cell Carcinoma | 3-45% | Yes |
| Disease | Coronary Artery Disease | 20-60% | Yes |
| Disease | Gout | 12-70% | Yes |
| Disease | Heart Attack | 25-70% | Yes |
| Disease | High Cholesterol | 12-50% | Yes |
| Disease | Hypertension | 10-45% | Yes |
| Disease | Inflammatory Bowel Disorder | 5-65% | Yes |
| Disease | Ischemic Stroke | 5-20% | Yes |
| Disease | Melanoma | 0-35% | Yes |
| Disease | Obesity | 12-65% | Yes |
| Disease | Prostate Cancer | 2-60% | Yes |
| Disease | Type 1 Diabetes | 10-55% | Yes |
| Disease | Type 2 Diabetes | 20-60% | Yes |
| Disease | Testicular Cancer | 0-55% | Yes |
| Mental Disorder | Major Depressive Disorder | 5-20% | Yes |
| Mental Disorder | Schizophrenia | 5-75% | Yes |
*See the section below for how to get access to these predictors
Ok, enough with the theory. How big of a benefit can you actually get from going through IVF and screening your embryos?
I’ll start with the one everyone always asks about: intelligence. How much can you boost your child’s IQ with embryo selection?
How much can embryo selection increase my child’s IQ?
First, there is no company that publicly offers embryo selection for intelligence. I have spoken with a stealth mode startup that offers selection for disease and non-disease traits, including intelligence. If you’re interested in selecting for non-disease triats, you can get in touch with them via Jonathan Anomaly, who knows some of the people working at the company.
Their current predictor correlates with measured IQ at about 0.4, which means they’ve likely compiled data from multiple sources to create it.
So how big would the gain be? Using some code from Gwern’s monster post on embryo selection for intelligence and some results from their calculator, I created the following graph:
It’s plausible that you would get up to maybe 40 euploid embryos if the mother is young and you do multiple rounds of egg retrieval. In that case, you could probably get a gain closer to 8 points. If the mother is older it will be less. There’s also a reduction in benefit if one of the parents is of non-european ancestry: likely around 8% for Ashkenazis, and 20% for east asians, and probably a similar or lesser reduction for Indians. I am uncertain of the reduction in gain for those of African ancestry, but it would likely be larger (perhaps 30-40%?).
This is an unfortunate side-effect of the fact that there aren’t enough non-Europeans in the large biobanks on which these predictors are trained.
There is significant room for this benefit to improve in the near future. The million veterans project in the US has whole genome sequences and ASVAB test scores for (you guessed it), a million soldiers. If researchers were simply allowed to use this existing data to create an intelligence predictor, the gain from embryo selectioin would increase to 8.5-13 IQ points and the racial disparities in predictor quality would mostly disappear. The marginal cost of this would be virtually zero.
This future increase in the efficacy of embryo selection has an obvious implication: if you freeze eggs or embryos now, you’ll have more embryos to pick from (since egg production declines with maternal age), and if you wait a few years to implant them, the expected gain from selection will be higher.
Disease Reduction
Unlike intelligence, there actually are several companies that offer polygenic embryo screening for disease risk. For this reason, I can tell you quite a bit about exactly how much you can reduce disease via embryo selection.
There are two main “categories” of disease risk to think about: monogenic disease risk (which includes diseases like Tay Sachs, Cystic Fibrosis etc), and polygenic disease risk, which includes heart disease, alzheimers, schizophrenia, diabetes and most others.
I’m going to focus on polygenic screening, since everyone has a non-zero risk of them, and embryo selection to reduce polygenic disease risk is already available in clinics.
The first company to offer this was Genomic Prediction. Orchid Health also finally offers polygenic embryo screening, though I believe they charge more per embryo. One other lab in China appears to have recently deployed a similar test, though it looks as though they screened embryos for type 2 diabetes risk exclusively, which is not a very sensible strategy in my opinion. And lastly, there is a stealth mode startup doing polygenic embryo screening. They offer screening for both disease risks and non-disease traits like intelligence. If you’re interested in contacting them you can reach them through Jonathan Anomaly.
Because I have little information about the predictor quality of Orchid Health or the stealth mode startup, I will focus on papers published by Genomic Prediction.
They use a pretty straightforward and simple method for determining an embryo’s relative ranking: each disease is weighted according to its impact on disability-adjusted lifespan. According to one of their recent papers, selecting embryos in this manner results in a fairly impressive reduction in disease risk across multiple conditions. Here’s a graph from one of their latest papers showing the expected reduction in the relative risk of various diseases from selecting the best of five embryos.
The figure above shows disease reductions for selection among 5 “pseudosiblings” of European descent (apparently there are not enough real siblings to train predictors on). My guess is the benefits will be reduced compared to those shown, perhaps by around 20%.
Note also that this doesn’t take losses from implantation or miscarriage into account. So you’ll need more like 7 or 8 euploid embryos to achieve gains 20% lower than this. But still, the benefits are reasonably strong.
The most amazing thing to me about the above graph is that selection seems to reduce EVERY disease in the index. One of the major concerns I hear raised about embryo selection is that there might be some “hidden downside” to selection. This graph seems to suggest that, at least so far as diseases go, that’s not much of a concern.
Another way of looking at the benefits of disease reduction is to look at how much the quality-adjusted lifespan of an average child born via polygenic screening would increase compared to one born without its benefits. Here’s their analysis of this framing:
Keep in mind, this is the pseudosibling benefit, so take what you see on the graph above and multiply it by 0.8 to get a more realistic number. Also, the benefit is not quite as large for non-European groups. It looks like the gain is reduced by about 20-30% for South Asians, 30% for Africans and 35% for East Asians.
Of course, this all kind of ignores the elephant in the room: many of these diseases have an average age of onset of 50-75. In fifty years the world is likely to look incredibly different. If humans are still around, it seems likely that we’ll have cures or at least very effective treatments for many of these conditions.
The exceptions are mental disorders like clinical depression and ADHD, which generally have an average age of onset before age 25, and obesity, which is now showing up more and more in childhood.
If Orchid Health is more expensive, why would anyone use their tests?
Orchid Health offers whole genome embryo sequencing. That means instead of just looking at ~800,000 spots in the genome, they look at about 3 billion.
This doesn’t make as big of a difference as you might think; most of the information we care about is already present in one of those 800,000 spots that companies like Genomic Prediction examine with their embryo testing. But whole genome sequencing has one specific advantage that companies like Genomic Prediction can’t replicate: they can detect de novo mutations.
De novo mutations are random genetic mutations that aren’t present in either of the parents. When you think of “random mutation and natural selection”, the “random mutation” part IS de novo variants.
We hear a lot about mutations conferring evolutionary advantages, both in science class when learning about evolution and in science fiction stories like X-men, but unfortunately most de novo mutations are just bad for you. Some of them are REALLY bad, as in your child will die quickly after birth or live a permanently stunted life.
Orchid’s testing finds one of these monogenic issues in about 3.7% of embryos. I’ll update this post later with more concrete info about the size of the benefit from having a child without those problems, but at a high level I think it’s likely worth the price if you can afford it.
Other Non-Disease Traits
Personality Traits
As with intelligence, there are no companies publicly offering screening for personality traits to the best of my knowledge. The stealth mode company has told me they may offer personality prediction at some point, though I am uncertain of their quality.
Predictors for personality traits such as conscientiousness and neuroticism are poor. This seems to be partly related to the lack of good data on such traits. A paper published in March of 2022 was able to explain 2% of the variance in conscientiousness and much less for others. There is significant room for improvement here, as most estimates peg the heritability of the big five personality traits at roughly 40-60%.
There are existing third-party services like Genomelink and SelfDecode which provide personality predictors. Unfortunately, they don’t have any published data on the quality of the predictors they use, and the anecdotes I’ve heard suggest that, at least for intelligence predictors, they are very poor. So if you’re looking for something that isn’t terrible, your best bet is to contact Jonathan Anomaly and hope the stealth mode startup offers something better.
Facial Features/Attractiveness
It would be nice (or possibly dystopian, depending on your views) if you could add physical attractiveness to your embryo selection criteria. More attractive people have higher lifetime income, better dating prospects, and seem to benefit generally from the halo effect.
To the best of my knowledge, no one offers this as a service right now, so unless the super secret startup offers it, you’d probably have to pay a researcher tens of thousands of dollars to develop a predictor for you.
The predictor might be halfway decent, though I don’t expect it to be as good as the ones we have for intelligence or most diseases. Here’s a meta-analysis of facial feature genetics from 2020 that found 203 genome-wide significant signals. If someone made a predictor using that paper it might be able to predict facial attractiveness reasonably well. However, there is clearly much room for improvement.
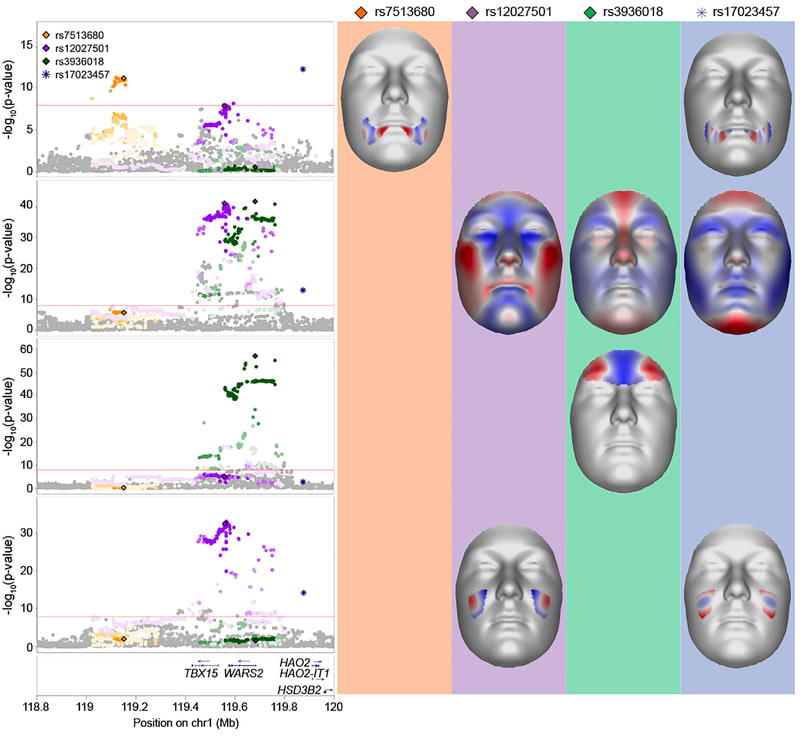
Is attractiveness a purely position good? That is to say, if everyone was made attractice, would society be better off? My guess is probably a little, but I haven’t done enough research into this topic to be sure.
If you want to see what selection on attractiveness looks like in the extreme, take a look at male birds of paradise. Female birds have been selecting mates based on their plumage and dancing ability for thousands of generations, resulting in some very beautiful, but very strange looking birds.

It is worth asking ourselves if dozens of generations of selection for attractiveness might result in similarly strange and pointless features whose sole purpose is to elicit arousal in members of the opposite sex.
And in the shorter run, selection for physical attractiveness probably trades off at least somewhat against selection for other features that have a greater benefit for society as a whole. So for now there is a non-zero cost to making everyone more attractive.
Still, from a purely selfish perspective, some level of selection for attractiveness makes sense. As with intelligence, no company currently offers the ability to select for physical attractiveness, unless you include selection for height, which already works extremely well. Your best bet is contacting Jonathan Anomaly to see if the groups he knows offer something. Or, barring that, you could hire a PHD student for 50k and pay them to develop a predictor for you.
Concrete Advice for Would-Be Parents
If you’ve read the sections above and you decided you want to do polygenic embryo screening, your primary objective should be to get maximum gain for minimum cost. This section is about how to achieve that goal.
There are two primary inputs that determine the effect size of polygenic screening:
The power of the polygenic predictors used to select an embryo for implantation.
The number of “achievable births”, meaning the number of children you would have if you implanted all of your embryos one by one.
Number 1 is largely out of your control unless your name is Gwern or you are a research scientist with access to a large biobank.
Number 2 IS within your control, at least to some degree. Here is a list of factors you might be able to change that will influence achievable births:
How many IVF cycles you go through
The IVF clinic you choose
The PGT lab used for aneuploidy testing
The age of the mother or egg donor at the time eggs are extracted (younger is always better)
The stimulation protocol used
The age of the father or sperm donor (younger is better)
Whether you choose to freeze eggs or embryos
Routine prenatal care that any good obstetrician will be able to tell you (though see Emily Oster’s excellent book if you want more details)
Why do these factors matter? Because at every step of the IVF process from initial consultation to birth, fewer eggs/embryos/pregnancies come out than go in. There’s a loss associated with each step, and the factors listed above have a large influence on the size of the loss. Here are some suggestions on how to reduce losses and costs:
Suggestion #1: Reduce uncertainty about how many euploid embryos you will produce
The single most important input into the “gain” equation is the number of mature eggs harvested per retrieval. However, this quantity has a very wide distribution. In conversations an acquaintance of mine had with egg donor clinics, they mentioned that some donors produce as many as 100 eggs per retrieval. A woman in her mid-40s with infertility issues might produce 3.
There are several heuristics you can use to reduce the uncertainty about how many eggs you are likely to harvest during an egg retrieval. Knowing these beforehand can help reduce uncertainty about the exact size of the benefits of polygenic screening.
The easiest heuristic to use is the woman’s age. As a general rule, I wouldn’t do IVF for the purpose of polygenic embryo selection unless the mother is under 38. Beyond age 38, the losses in the IVF funnel are just too steep to justify the pain and expense, unless you plan to use donor eggs.
Another lower-cost but not free thing you can do is assess your likely egg production by undergoing the very first part of IVF; a consultation and ovarian ultrasound. This ultrasound is performed right at the very start of the IVF process and usually costs less than $1000. Ovarian reserve and antral follicle count are strongly correlated with the expected number of mature eggs you or your female partner will produce after hormonal treatments. If you’re willing to do embryo selection in theory if the gain is large enough, this can be a relatively inexpensive way to reduce the uncertainty about the benefits of the procedure.
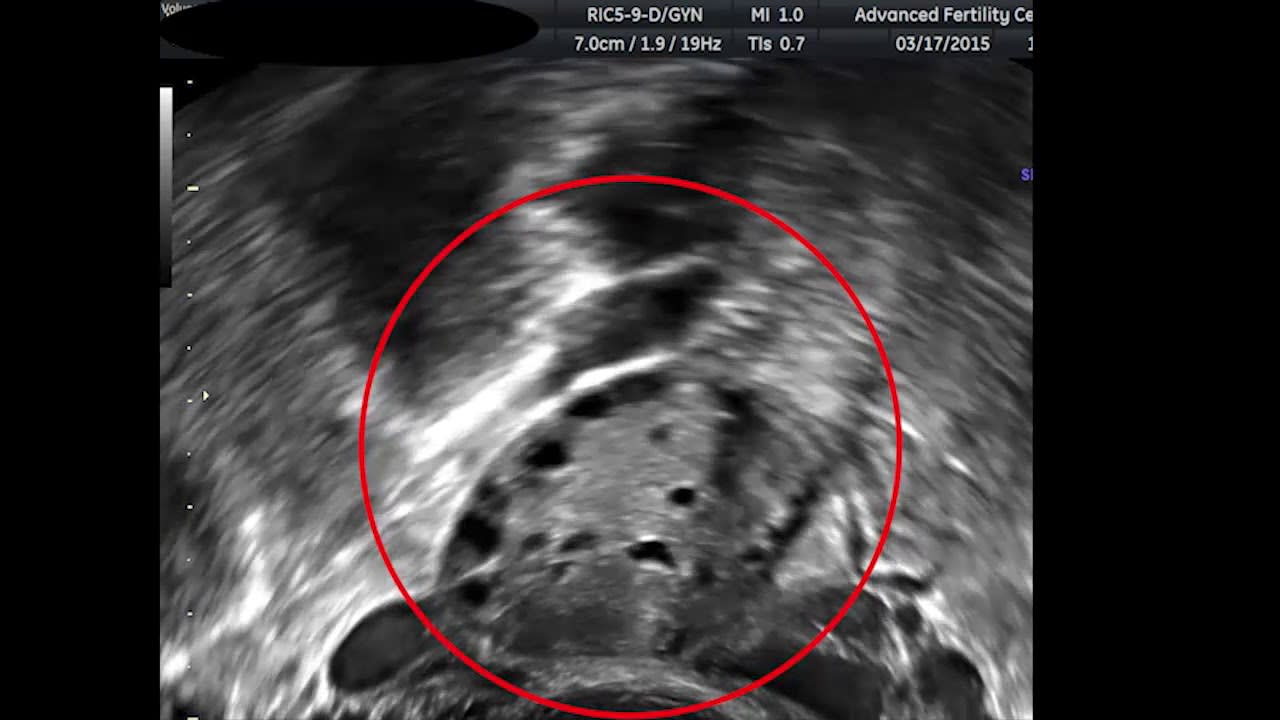
What is really needed here is a tool that allows one to see the expected gain across a variety of traits given an antral follicle count. The data necessary to do this research is present in the CDC’s NASS database, but I don’t yet have access. I hope to do this for a future research project. Until then, here’s a table showing roughly how your odds vary as a function of antral follicle count.
Suggestion #2: Use my table to pick a clinic
I have spent an embarrassing amount of time on a research project to rank every IVF clinic in the US from best to worst. I compiled this list using data from the CDC’s NASS database, which has information about clinics going back to the mid-90s. I believe I am the first and only person in the world to do this. If you’re curious about a clinic that isn’t on the last, feel free to reach out to me and I can give you the numbers.
A somewhat boring explanation of the research I did (skip this if you just want results)

Clinics are ranked according to their cumulative live birth rate per intended egg retrieval among patients using their own eggs (not donor eggs). In simple English, that means we’re looking at what percentage of women who started hormone treatments actually delivered at least one child.
In an ideal world I’d give you clinic rankings based on the number of expected births per retrieval. But without access to intermediate outcome data from NASS, this is impossible. I plan to apply for access eventually, but in the meantime I think live birth rate is probably quite a good proxy, and clinics with very high live birth rates are likely to be able to produce more achievable births than those with lower live birth rates.
I’ve taken care to control for various factors that could confound the analysis. Some clinics attract a larger proportion of younger patients, who have better prospects than older cohorts. Some clinics attract a large proportion of patients solely interested in freezing eggs or embryos for some later treatment. Some clinics have a very small number of cycles each year and can score very well or very poorly depending on the luck of the draw. I’ve controlled for all of these confounders in my analysis.
The one big thing I didn’t control for was the percentage of patients presenting with a given infertility diagnosis. I attempted to to this in an earlier version of the project but had weeks and weeks of nightmares trying to find some defensible way to deal with the large amount of censored data present in spreadsheets. Supposedly the CDC censors these values to protect patient privacy. This justification is obviously nonsense; they still have uncensored values from before 2018 on their website. I emailed to ask if I could apply for some kind of special access as a researcher and was denied.
I tried to work around these missing vaues but eventually I simply gave up. I had to make too many unjustified assumptions to compute clinic rankings, and ranking was highly variable depending on which assumptions I made.
So this final analysis controls only for maternal age, use of patient’s eggs (vs donor eggs), percentage of retrievals conducted with the intention to freeze embryos or eggs (obviously those people aren’t going to have a baby), and some bayesian averaging sprinkled in on top to differentially bring clinics with low retrievals/year more towards the mean of all clinics.
I plan to actually publish my results in proper academic setting at some point, so this post contains only the headline numbers.
Without further ado, here are the top 25 IVF clinics in the US as of 2020.
The best IVF clinics in the USA
| Clinic Name | Adjusted Live Birth Rate | Clinic State | Phone Number |
| Carolinas Fertility Institute | 0.516123 | North Carolina | (336) 448-9100 |
| The Georgia Center for Reproductive Medicine (no website) | 0.490339 | Georgia | (912) 352-8588 |
| Reproductive Gynecology & Infertility-Westerville (Columbus location) | 0.482645 | Ohio | (614) 895-3333 |
| Reproductive Medicine Associates of New Jersey | 0.458511 | New Jersey | (973) 971-4600 |
| Center for Reproductive Medicine, Advanced Reproductive Technologies | 0.457538 | Minnesota | (612) 863-5390 |
| Missouri Fertility | 0.456779 | Missouri | (573) 443-4511 |
| Spring Fertility (San Francisco location) | 0.446066 | California | (415) 964-5618 |
| CCRM Boston (main center in Chestnut Hill) | 0.439929 | Massachusetts | (617) 449-9750 |
| SpringCreek Fertility (Dayton location) | 0.437458 | Ohio | (937) 458-5084 |
| Duke Fertility Center, Duke University Medical Center | 0.419579 | North Carolina | (919) 572-4673 |
| New Direction Fertility Centers | 0.413924 | Arizona | (480) 351-8222 |
| Shady Grove Fertility Colorado | 0.411165 | Colorado | (720) 704-8221 |
| Center for Advanced Reproductive Medicine | 0.409579 | Kansas | (913) 588-2229 |
| Fertility Center of the Carolinas | 0.406704 | South Carolina | (864) 455-1600 |
| Fertility Center of San Antonio | 0.403583 | Texas | (210) 692-0577 |
| Baystate Reproductive Medicine | 0.403039 | Massachusetts | (413) 794-1950 |
| University of Iowa Hospitals and Clinics, Center for Advanced Reproductive Care | 0.401587 | Iowa | (319) 356-8483 |
| Carilion Clinic Reproductive Medicine and Fertility | 0.400715 | Virginia | (540) 985-8078 |
| Advanced Fertility Center of Chicago | 0.399771 | Illinois | (847) 662-1818 |
| Shady Grove Fertility-Richmond | 0.394682 | Virginia | (804) 379-9000 |
| Fertility Center of Southern California | 0.391824 | California | (949) 955-0072 |
| Northern California Fertility Medical Center | 0.390555 | California | (916) 773-2229 |
| The Nevada Center for Reproductive Medicine | 0.390344 | Nevada | (775) 828-1200 |
| Center for Advanced Reproductive Services (Farmington Location) | 0.388567 | Connecticut | (844) 467-3483 |
For reference, the average adjusted live birth rate for all clinics nationwide was 0.278.
How predictive are an IVF clinic’s past success rates of their future success rates? Here’s a graph showing how well a clinic’s 2017 live birth rates correlated with their 2020 live birth rates after adjusting for the confounders I mentioned above:
The overall takeaway here is fairly clear: by selecting one of the top 25 or so clinics in the most recent year, you can increase your achievable births by perhaps 20-30%.
I have been working on a more advanced version of the model used to produce the results above which makes more efficient use of crappy, censored data and shows results for 2021. I also plan on releasing a free app to the app store making all of this data much more usable. This post will be updated when it goes live.
Suggestion #3: Use a good PGT lab
TL;DR Make sure you use Orchid Health or Genomic Prediction for aneuploidy testing. They very likely have lower false positive and false negative rates for embryo aneuploidy when compared with other PGT labs.
It is a little known fact that there is a significant difference in aneuploidy false positive and false negative rates between PGT labs. To the best of my knowledge, there have been no randomized control trials comparing PGT labs. The best we have are independently conducted retrospective cohort analyses.
Unfortunately most of these analyses do not disclose which labs are being analyzed, making them completely useless for paients. However, I happen to know which clinics are which for one particular study submitted to ASRM in 2021. In this study, clinic A is Igenomix, clinic B is Genomic Prediction, and Clinic C is Cooper Genomics.
There’s a lot of sort of random statistics thrown out in this presentation, but I’d like to focus on those most relevant to achievable births: aneuploidy rates, pregnancy rates and miscarriage rates (which they break up into early and late miscarriages in the study)
If you watch the video, you’ll see that “Lab B (AKA Genomic Prediction)” is either as good or significantly better than the other two labs in the study across virtually every metric. If you add up the impact of these metrics, here’s what the expected number of achievable births look like for each clinic from the study:
I don’t know of any direct comparisons of Orchid Health with other PGT labs, but my guess is their false positive and false negative rates are at least low as those of Genomic Prediction. They use whole genome embryo sequencing, which means they retrieve a gigantic amount of data from each embryo. It’s likely this amount of data reduces testing errors compared to low density NGS sequencing, but I am not certain about this.
Suggestion #4: If possible, freeze embryos instead of eggs
This one is short and sweet: if you have a choice, freeze embryos instead of eggs. At a good storage facility using vitrification, about 90% of eggs will survive cryopreservation. At that same storage facility, 99% of embryos will survive. So if you already know who you want kids with, freeze embryos instead of eggs for an easy 10% boost in achievable births.
Suggestion #5: Freeze eggs or embryos as soon as possible
Since expected gain increases the more achievable births you have, all tips for maximizing it revolve around increasing the number of euploid blastocysts you can produce during IVF. You can pick a good clinic, use a good PGT lab, freeze embryos instead of eggs, and follow good prenatal care guidelines. But at the end of the day, the single biggest input variable into the “gain” equation is the age of the mother.
Here’s a graph from another research project I did showing the relationship between maternal age and number of eggs retrieved at three different clinics
You can see there’s more or less a linear decline in expected egg count per retrieval as a function of maternal age. It’s much the same story for expected zygotes and blastocysts; a linear or even exponential decline as a function of maternal age.
If you decide to do polygenic embryo screening, the sooner you start the process the better.
A compendium of other advice
When choosing a clinic, there’s several things you need to ensure they are OK with before you agree to become a patient
You need to be able to send your embryo’s biopsies to a lab of your choosing, or they need to already be working with Genomic Prediction or Orchid Health.
If you want to screen for anything other than the diseases offered by the above companies, the clinic must to be willing to implant an arbitrary embryo of your choice.
Make sure that the clinic you choose either has reasonable embryo storage costs or will let you ship your frozen embryos to a facility that does. Some clinics charge up to $1500/year for embryo storage and will raise the price on you as time goes on. Cheaper clinics charge under $1000/year for storage (some as little as $500)
Read Emily Oster’s excellent book about the things you should and shouldn’t do before, during and after pregnancy. Seriously, Oster is excellent and enjoyable to read. Ex: gardening is dangerous for pregnant women due to soil microbes but <3 drinks per week seems to be completely fine. The one possible exception to this is advice about alcohol consumption, but the science there is very complicated and confounded by selection effects.
If you’re a man over 30, consider freezing your sperm. De-novo mutations disproportionately cause conditions like cognitive deficits, severe autism and other serious conditions, and most of these mutations come from the man’s sperm.
The IVF Loss Funnel
Ok, if we put together all the data above, how many live births can you get per egg retrieval?
Naively interpreted, the above graph would imply that an average IVF cycle would only yield a single achievable birth on average. This is true! The average IVF patient is a 36 year old woman with significant fertility issues, so it’s not particularly common for such individuals to have more than one birth per egg retrieval. In fact, over half of IVF cycles do not result in live birth. The average is dragged up somewhat by the fact that some women are able to have multiple children from a single egg retrieval.
But what if you choose a better IVF clinic and a better PGT lab than average? What if you and your partner have no known infertility issues and your female partner is younger than the average 36 year-old IVF patient (say 32 for this example).
In that case, we’d expect the graph to look something like this:
Looking better! With a younger mother, a top-tier clinic, and no history of infertility, roughly 5 achievable births per retrieval is possible.
How about in the best case scenario? Assume the following:
The mother is at peak fertility (early to mid 20s)
Egg retrieval is performed using conventional IVF hormone treatment
The father is young-ish (under 40)
Neither parent has any infertility issues
The parents use a top-tier clinic that is very good at culturing eggs into blastocysts
They use a top-tier PGT clinic with a very accurate aneuploidy test
The eggs are fertilized immediately and the resulting blastocysts were frozen
In that case, things start to look A LOT better.
The numbers above are based on a combination of sources including SART data on miscarriage and transfer loss rates, podcast episodes, publicly available data from egg donor clinics, and my own knowledge of the IVF industry. I suspect that it may be somewhat conservative for couples without infertility since I have used the infertile transfer and miscarriage rates. But they should nonetheless give a fairly accurate view of the loss funnel.
One last topic I shoud address; how much will all of this cost?
How much does IVF and PGT cost?
IVF is expensive. To do polygenic embryo screening you’ll need to pay for a consultation, ultrasounds, transportation to and from the clinic, IVF services like lab techs, medication, pre-implantation genetic testing, and data analysis services to select for non-disease traits.
I’ve called a couple of dozen top-tier IVF clinics on the phone to ask about prices. I’ll give you a general cost estimate based on those calls:
| Service | Price Range | Modal Price |
| Consultation | $50-550 | $300 |
| Follicular Ultrasound | $150-500 | $400 |
| Medication | $3000-$6000 | $4000 |
| Egg retrieval (not including transfer) | $6000-20,000 | $14,000 |
| Embryo Transfer | $3000-$6000 | $4000 |
| PGT-P | $1500-5000 | $1000 + $400/embryo |
| Selection for intelligence, height, etc | $15,000-$50,000 | Best guess: $20,000 |
| Total | $9000-$35,000 | $26,500 + intelligence screening |
This process is not cheap. If you want to do two egg retrievals and select for intelligence you’re looking at a minimum of $50k and possibly much higher depending on what you’d like to select for. I sincerely hope we can bring the price of these services down in the coming years.
TL:DR
Polygenic embryo selection can currently increase your child’s quality-adjusted life expectancy by 1-4 years, decrease their risk of various chronic diseases by 10-60%, increase their IQ by 2-8 points, increase height by up to 2.25 inches, and moderately improve other traits. The exact gain you can expect to get for each of these traits varies depending on the genetic correlation between the traits, the number of embryos you have to choose from, and the strength of the predictor used to select embryos, as well as simple luck. Subsequent children will see a somewhat smaller but still positive benefit, though for every child to benefit you will need at least 3x the number of euploid embryos as you want children.
To get these benefits, you will have to go through IVF and genetic testing of your embryos, which will cost $20k-$60k (and perhaps more depending on whether you want custom testing) and require the female partner to take 2-6 weeks off work.
You can increase the expected gain by choosing a good IVF clinic, choosing a good PGT Lab, freezing embryos instead of eggs, and beginning the process as soon as possible since younger mothers produce significantly more eggs than older mothers.
The IVF process is not particularly pleasant, and is expensive to boot, if you and your partner are willing to put up with the discomfort and expense it you can give your children advantages that are impossible to get any other way.
If you freeze embryos now, the expected gain will increase over time as the genetic predictors used to select embryos improve, and the panel of traits which you can select for will also increase.
There are technologies on the horizon that will allow for significantly greater gains across all heritable traits, making possible gains of 4 or more standard deviations across multiple traits simultaneously.
If AI doesn’t destroy the world first, the next 30 years will likely see the greatest crop of geniuses and athletes the human species has ever produced. If we are wise and select for traits like kindness, altruism, and happiness in addition to health, attractiveness and intelligence, the children born with these benefits may be able to guide the human species through the incredible upheaval and instability we are likely to see over the next century.
- Significantly Enhancing Adult Intelligence With Gene Editing May Be Possible by (12 Dec 2023 18:14 UTC; 404 points)
- Twiblings, four-parent babies and other reproductive technology by (20 May 2023 17:11 UTC; 188 points)
- Four visions of Transformative AI success by (17 Jan 2024 20:45 UTC; 112 points)
- Reply to a fertility doctor concerning polygenic embryo screening by (29 May 2023 21:50 UTC; 58 points)
- Intelligence Enhancement (Monthly Thread) 13 Oct 2023 by (13 Oct 2023 17:28 UTC; 51 points)
- Concrete positive visions for a future without AGI by (8 Nov 2023 3:12 UTC; 41 points)
- 's comment on A moral backlash against AI will probably slow down AGI development by (EA Forum; 1 Jun 2023 14:30 UTC; 8 points)
- 's comment on Cause area: Short-sleeper genes by (EA Forum; 6 Jul 2023 16:05 UTC; 4 points)
- 's comment on Are c-sections underrated? by (7 May 2023 17:08 UTC; 4 points)

Thank you, this is a great post. A few questions:
You say “see below for how to get access to these predictors”. Am I understanding right that the advice you’re referring to is to contact Jonathan and see if he knows?
I heard a rumor that you can get IQ out of standard predictors like LifeView by looking at “risk of cognitive disability”; since cognitive disability is just IQ under a certain bar, this is covertly predicting IQ. Do you know anything about whether this is true?
I can’t find any of these services listing cost clearly, but this older article https://www.genomeweb.com/sequencing/genomic-prediction-raises-45m#.ZFqXprDMJaR suggests a cost of $1,000 + 400*embryo for screening. Where did you get the $20,000 estimate?
Yes. My understanding is he knows some groups that have a working IQ predictor and are accepting customers.
Genomic Prediction no longer offers an intellectual disability predictor. They got huge blowback when they first released that predictor and removed it from their traits as a result.
I do not believe that you’d expect to get much of an IQ bump from selecting against disease risk either. My guess is less than 1 point from 10 achievable births.
Sorry, I should really go back and edit the post to make this clearer. To the best of my knowledge Genomic Prediction (and possibly Orchid) are the only companies that can genotype your embryos with reasonably good quality and will give you the raw data. This is the part that (probably) costs about $1000 + 400 per embryo.
You then have to take that raw data to a third-party service (probably one of the groups that Jonathan knows) and ask them to predict the IQ and/or other traits. I don’t know anything about the groups, but I’d be shocked if they’re doing this for free. So they will charge an additional amount, which is where my estimate of $20k came in. I don’t actually know anything about their prices so that’s a complete shot in the dark for what it costs.
But given it’s a low-volume service at this point, my guess is it’s quite expensive.
Thanks!
Does anyone here have qualms about the moral status of the embryos that are discarded in this process? I’m aware of the OP’s views on the issue, and I recently addressed them elsewhere, but I’m curious about the average viewer of this page.
Emphatically no. They are primarily potential lives, but they need their mother’s womb to achieve that potential, and they can’t all have that. Those simple brains have no more inherent moral worth than an insect with a similar number of neurons. Only one life can come out of this process. Whether that one is chosen by chance or choice, the one resulting life has the same moral worth.
The statement that they are potential lives is incorrect. An embryo is already alive and, since it has continuity through time with an adult human being (obviously actual living human), it has human identity as well. Therefore, it is a living human being.
“Only one life can come out of this process” is also incorrect. This is like having 4 teenagers and choosing 3 of them to be shot, and then concluding that “only one adult can come out of this process, therefore the 3 teenagers are merely potential lives and can be destroyed”.
Why would inherent moral worth depend on the number of neurons or complexity of the brain?
My God… The discarded embryos are human beings with all the associated moral worth. The procedure described in the post does not eliminate diseases or increase the iq of a child. It merely kills the humans who are more likely to develop a disease or those who are likely to have lower iq.
This is evil.
Hey @GeneSmith, really appreciate you putting this together. I wanted to throw in a few thoughts regarding monogenic disease (the disclaimer/context here is I lead engineering at Orchid, and we’ve put a lot of thought into our monogenic embryo screening).
The 1% risk of monogenic disease you cite above is pretty misaligned with most estimates of monogenic disease. It may be an old reference, or filtering for only catastrophic disease, but common estimates for monogenic screening yield in adults are between 3.5% and 18%:
BabySeq found 18 out of 159 infants (11.3%) with diagnosable and actionable disease variants.
DiscovEHR found 3.5% of adults with pathogenic actionable variants
Vassy et al., 2017 found that of 50 healthy individuals who were sequenced, 18% had a pathogenic or likely pathogenic variant associated with monogenic disease.
(we’ve written a bit more here about how the screening we do translates into a % of embryos with findings).
It’s important to remember that carrier screening is very limited and conservative, generally flagging only recessive variants strongly associated with health conditions (deafness, etc) on a limited list of genes. But in reality there are a large number of low-penetrance, or moderate pathogenicity, pathogenic dominant variants that a WGS screen (like the above studies, and our PGT-WGS) surface.
The GC and reproductive health community is very conservative, and defaults to not scaring patients (as they see it) and not trusting them with probabilistic outcomes, so you end up with pretty conservative carrier screening panels. But this is the same instinct that makes them reflexively opposed to PGT-P! So if we’re talking about monogenic vs polygenic screening it’s reasonable to look at the whole universe of risk alleles (which may have a 3x elevated risk of disease, or a 10% risk of disease) rather than the limited carrier screening lists.
And what we see in practice aligns with these numbers. For example, we have a case whitepaper here (more soon) where we detected a pathogenic variant linked to cardiomyopathy in embryos from an IVF cycle; when we consulted with the patients, it turned out the male partner was on medication for dilated cardiomyopathy! The dominant variant wasn’t on the carrier screening panels, but in practice finding a variant like this is more informative than a 99% PRS for heart disease which may only translate to a 2-3x risk of disease.
I don’t at all mean to cast shade on polygenic screening, because of course we offer PGT-P (and raw data exports), and if you’re in the 90% of couples without a monogenic finding, it’s absolutely the best way to move the needle. But I do really think that:
Monogenic screening, even today, has a very high ROI on future disease prevention. In aggregate rare disease costs something like $1 trillion a year, most of that is genetic, and monogenic screening even today can catch a real % of that (I’m spitballing ~50%, but the ROI even there is ~15x so there’s a lot of room for ballparking).
As large biobanks come online, we are going to see a lot more monogenic pathogenic risk alleles identified. Many of these variants are individually extremely low prevalence and we simply aren’t seeing them linked to disease until we have biobanks with 1mm+ individuals with genotypes linked to health data.
Very happy to go into any of this in more detail, and of course really appreciate the work you spent putting this guide together : ) Most patients have no idea where to start on embryo screening and your guide is an incredible reference for orienting them.
Thanks a lot for the comment. I’ll amend the post with some of this information in the next week. If your numbers are correct (and I have no current reason to doubt them, that substantially increases my estimate of the effectiveness of whole genome embryo sequencing.
I’ve been meaning to write a whole post about the different screening companies but a combination of little time due to starting a new company and a lack of clear data have preventing me from doing so thus far. With this information I might reconsider.
One more thing I’d like to ask at some point is whether you’re going to publish the AUCs of all the predictors in your panel within some reference population. That would be extremely helpful for patients trying to compare Orchid vs Genomic Prediction or any other company.
You are recommending “Expecting Better” by Emily Oster for prenatal care?
Isn’t that the author who claims that pregnant women can have “1 to 2 drinks a week in the first trimester (!)” and one drink daily (!) afterward with no worries?
That is terrifyingly risky advice, contradictory to all major guidelines (CDC, WHO, American Pregnancy Association, the UK Health Ministry, Fetal Alcohol Syndrome advocacy groups...). There have been significant mental changes in babies from quantities as low as that. https://ajp.psychiatryonline.org/doi/epub/10.1176/appi.ajp.2020.20010086 Whether your kid will be one of them is a matter of luck, depending on factors we do not yet understand, but it is really not a bet I would take, and certainly runs counter to the aims of polygenical screening.
I’m a little too tired to read through the study you sent me. I suppose it’s possible she either got alcohol wrong or that new studies have come out since the book was published which should change the conclusions.
I would note that it’s very hard to assess the impacts of drinking in the current environment because women who adhere to health advice about not drinking are probably going to pass on genes to their children that make them less prone to the very neurodevelopmental disorders the studies purport to measure.
You could do a study where you find women who you think are at high risk of drinking during pregnancy and then perform an intervention to encourage some of them not to drink. But given that ERBs adhere to a strict Copenhagen interpretation of ethics I doubt such a study design would ever be allowed (even though conducting it would improve children’s health if alcohol does indeed have a negative impact).
It stands out to me that she contradicted guidelines by all major agencies and metareviews on this issue, specifically reassuring mothers that it is safe, when so much data points to extreme harms in large quantities, and to the fact that these harmful correlation lower, but stay significant all the way to women who have one single drink. Also that she stuck with it despite having this pointed out to her. It is possible that there is another explanation for the incurable brain damage, and more research is certainly a good idea, but that is a far cry from denying such a highly plausible risk to encourage an behaviour that is completely unnecessary. The fact that she does in this situation has me sceptical of all the rest she recommends, too.
I am also not sure whether your correlation idea holds. Many documented cases occur early in the first trimester, because the woman in question is unaware that she has gotten pregnant, and quits as soon as she learns. I doubt that a woman who believes she is not pregnant having a single glass of wine is indicative of anything else you’d expect to be highly correlated with the characteristic fetal brain damage and facial changes we then encounter. The fact that this has become so common that we no longer even recognise those facial changes indicating the brain damage as strange at all is frankly frightening.
Importantly, the negative impact of even very little alcohol on the fetus outweighs the IQ benefits you would be spending 20-100 k on.
FWIW, here’s a Cochrane review on RCTs of such encouragement designs. It’s basically a failed meta-analysis in that the data is too spotty to make any conclusions. But it shows at the very least that such studies have been done before. An encouragement design in a population with high alcohol use seems most promising for figuring out the causal effect on fetuses, as you say.
(Another recent meta-analysis on the same question finds a slight decrease in preterm birth in the alcohol education group, but this is based on just three studies and marginally significant so I’d say it’s still uncertain.)
These seem most consistent with the effect of light drinking being small or zero, though it’s hard to be certain with the sample sizes given and the difference in study designs.
I wish someone would do a large, well-designed, well-run RCT of an encouragement design so we could have a definitive answer to this question.
Great post. Thank you. Fertility doctor here and a supporter of ART (assisted reproductive technologies) in general. A few thoughts (although you touched on a few of these below, worth emphasizing in my opinion):
PGT-P has not been validated yet, which may take decades to do, if ever.
The science in terms of GWAS isn’t quite there yet IMHO—we don’t know all the genes that are important for most traits and we may be inadvertently selecting against some desirable traits, for example.
Comparing clinic success rates using CDC data is imperfect because of different patient characteristics, patient selection, and reporting bias.
IVF pregnancies carry a significantly higher complication rate (hypertensive disorders, preterm birth, placental abnormalities, etc.) compared to spontaneous pregnancies—unclear if this is due to IVF or underlying infertility diagnosis.
The risk-benefit calculus of PGT-P is going to be different for a couple who already needs to do IVF anyway to have a baby (low additional risk/cost) compared to a couple doing IVF just so that they can do PGT-P (higher additional risk/cost).
IVF is notoriously inefficient at present. Depending on female partner age, each cycle may yield only very few embryos making the benefit and utility of PGT-P limited. It may not be practical, safe, or financially feasible to do multiple cycles of IVF to increase the cohort of transferable embryos.
IVF is expensive and often not covered by insurance which creates access disparities. PGT-P would exacerbate these disparities in access. This is not unique to IVF I realize.
Slippery-slope eugenics and discrimination are real ethical concerns that would need to be mitigated.
In-vitro gametogenesis (IVG) would be a game-changer. The utility of PGT-P would be greatly enhanced if suddenly you had thousands of eggs and hundreds of embryos to select from.
I made my reply to your comment into a standalone post
I think you are not taking the eugenics and discrimination concerns seriously enough.
I think few people disagree with the idea of selecting the embryo with fewer avoidable diseases that would involve severe suffering; I do not think alzheimers makes humanity better. But there is a slippery slope towards a scenario where people select for sex, skin (and hair and eye) colour, not being queer, not being neurodivergent… and I do find that a dystopian scenario where we would lose something valuable that enriches our world. It makes me worry about and for the people who would opt out of being fixed, or having their children fixed (or rather, discarded early and replaced). I wish I didn’t have the genetic tendency towards disease that I unfortunately have, and that causes me a lot of suffering. But I assure you, if my parents had gotten to pick, they would also have made sure that I am not gay. Or tall. Or enby. Or have ADHD and autism. Things that were challenges, to be sure, but that I do not see as a net negative. I also know that both my parents found it disagreeable that I am highly gifted, and smarter than them; my father explicitly considers it a disease state and unpleasant complication, and would certainly have selected against it. I am very glad that he could not.
We also already have a scenario where it is incredibly difficult for a poor person to have proper upwards social mobility. If their competitors are literally superior from birth, prior to also getting their personal tutoring and private schools and trust funds and brain implants… at some point, no amount of hard work will make you competitive anymore, the different classes will become insurmountably separated and fixed. Not because the rich parents want this—they just want the best for their children, who wouldn’t. But if access to this tech is not fair, and its legitimate usage is not carefully reflected and set, the changes could be dystopian indeed.
This tech is transformative. I see how it could make humanity better, more just, more diverse, happier… but also how we could end up with an untouchable elite pushing for a narrow norm in which people like me, and many of my friends and loved ones, would have been sorted out for traits that we are glad we have.
Yes, this is actually a fairly common critique of embryo selection. One useful intuition pump I’ve found helps me think about it is the reversal test; should we make people sicker or more mentally distraught to enrich the world? It’s a bit odd to imagine that evolution somehow put us at the perfect equilibrium where any increment or decrement in mental illness rates would result in a worse society. It’s especially odd to think that since evolution doesn’t care at all about either of those things except insofar as they affect inclusive reproductive fitness.
Also, my experience so far just talking to people makes me think parents are going to have different priorities regarding the traits they select for.
I’m sorry about your parents. That sounds like an unpleasant experience.
I don’t think the thought experiment of “erasing” someone like you from existence is really a very good test of the morality of embryo selection. You are a person with decades of memories and ties to the community of people around you. In my view the morality of “erasing” you feels a lot different than making a choice between two embryos. Unless you believe in souls or something, an embryo is almost pure genetic potential. It has no internal organs, let alone a brain. Even the placenta hasn’t formed yet.
I would point out that all the dynamics you described are already true to some degree; there are some people born with such extreme genetic disadvantages (through a combination of parentage and bad luck) that there are some paths in life simply closed to them.
Of course embryo selection will increase variance, so your point is still well taken. I’ve spent a fair amount of time thinking about this and the obvious solution here is just to work very hard to make this technology cheaper and better. If we make enough progress on that front then we can just have the government subsidize the technology and give free access to anyone that wants it.
Inequality WILL still increase in the meantime, but there are some dynamics that I think help us here:
There is a ceiling on improvements through embryo selection or editing. That limit is determined by the amount of variance in the human gene pool. The ceiling is very high, but its existence makes it plausible that some people will get there first and others will catch up
To push beyond that ceiling you’ll either need to generate new genetic variants and test them out in people. This will require the cooperation of a very large number of people. To make good predictors today, you need literally a million people or more in a database. To a first approximation I would guess you’ll need that many if you want to test out a ton of new genetic variants and have enough statistical power to distinguish true positives from false positives.
The main way rich people can get an advantage in embryo selection is by harvesting more embryos or getting access to better predictors. The predictors are all made from huge databases, most of which are public. So it’s unlikely rich people could maintain a monopoly on the best predictors. Also, it’s hard for them to get a big advantage by selecting from a lot more embryos. You can of course pay to use a better clinic, and you can pay to go through more egg retrievals to harvest more embryos. But there are steeply diminishing returns; you’re still sampling from a normal distribution. The expected maximum value of N samples from a normal distribution is sqrt(ln(N)). That’s an INCREDIBLY slow growing function. If you go from 10 embryos to 1000, the benefit only increases by 70%.
Will rich people still have an advantage? Yes. But genetic enhancement does not have the same runaway “intelligence explosion” dynamics that AI does.
Not necessarily disagreeing with your main point (“discrimination concerns”), but I want to note that the status quo is currently that an alien god gets to choose those traits. Given the option to wrest (more) control away from natural selection, specific humans, or even humanity collectively, might indeed choose to exercise that control in even worse and more horrifying ways than evolution.
So “even more horrifying” is a way things might go, and it’s worth weighing that in a cost-benefit analysis. But I just want to note that, as a transhumanist, I regard the status quo, of ceding humanity’s collective heritage to an unthinking and unfeeling alien optimization process, to already be pretty horrifying.
Partially, I’m horrified at some of the actual outcomes it produces, but I also have a larger philosophical discomfort with trusting something as important as the process by which humans get created to such an alien process, in a universe that (aside from humans themselves) is so unthinking and uncaring and unfair.
I think I have a different perception of nature, and a much more positive one.
With every single life form I see, myself included… I look, and think, your parents survived until they brought children. And their parents survived until they brought children. And their ancestors. And their ancestors. All the way back, for 3 billion years, a single unbroken chain beginning in the first clump of life that hung on, that survived, that hung on, grew, diversified, learned, evolved, a chain of survivors longer than I can conceive. What lives in me now, what lives in all of us, went through this incredible forge, and survived. It has me mesmerised. I look at every strange, random thing, and think, this chose itself, it proved itself, it brought us here.
I look at my body and think, this is a freaking self-assembled nanomachine. I started with two cells, one in the literal nanorange, the other so small it can barely be seen, and that only because it already carried some building blocks for cannibalising later. And they self-assembled into an entire human being, every single step depending on the next, and enterprise that seems insane, and yet here I stand, and this body works. I breathe, I move; my heart cells began beating in the sixth week of pregnancy, and they have never for a single minute stopped since. I can consume practically any organic substance, and my body will disassemble it, and then reassemble it into more of me (how crazy is that?), while breaking down invaders and poisons in the process. I can be cut, and my body will automatically begin self-repairing. I can be invaded by hostile life forms, and have them eliminated. You can destroy a chunk of my brain, the very substrate of my mind, and it will reroute, rebuild, recover.
I walk into a forest, and I am overwhelmed by the beauty, the complexity, the balance of it. I look at plants and think, fuck, this thing is literally made of air. It stole the energy from the sun, grabbed some micros from the soil, and literally built itself out of thin air.
Every time in nature that I encounter something that seems destructive, I see new beginnings emerge from it, nothing wasted, everything re-entering the cycle. The forest fire that germinates the seeds. The flooded areas that have amphibians lay their eggs. The tree struck by lightning that becomes a home to birds and bats.The elaborate net of complex processes that attenuate each other, resilient to catastrophes, rebuilding. I watch the Chernobyl site recover into a jungle, and am in awe; a place which humans did their best to destroy, and nothing to rebuild, just retreated from… and that rebuilt itself. I see how life arounds me is in a constant process of adapting, shifting, making use of new opportunities, evading new threats.
I adore how even the most brutal and horrific thing has a logical explanation—not a kind one, but a true one. I look at this vast web that plays together like a clockwork more intricate than any we have ever made, but a clockwork that is never done, in which an item can be smashed, but then, the whole begins to shift, to change, and a new balance emerges. I laugh and weep at the beauty of it.*Life can be so cruel, so painful, but it is the reason we have sentience at all, feel anything at all. My very joy right now is the result of evolution, selected for its adaptive advantage.* Out of this dead planet, this broiling chaos, we got life, we got sentience, we got minds that can recognise themselves, and think about all this. Not given by a kindly God, or even an evil or uncaring one, but fought for, torn from death and against the chaos.
There is no God, not even an alien one. Noone wanted us here. Noone picked us. Life picked itself. It held together against entropy. Without breaking a single physical law, without magic, without cheating, we still managed to break the consequences and flaunt the result; I see it in every bird that, instead of falling like a rock, soars to the sky. I see it in humans who escape their gravity well. This overwhelming sense of survival, of defiance.
I’m a transhumanist, too. But for me, that does not feel like a contradiction to and opposition of natural life. It feels like its continuation. Something true to the very character of life, to resist, to become something better than there was, to forever change and adapt, to survive, to diversify, to thrive.
But for me, it is also entangled with the other lessons of life; that we are part of a complex whole that exists not for us, but for itself, our origin, and our home, a vast web of interdependence, carefully synchronised.
There are reasons for why nature is the way it is, and while it is not perfect, we need to think very, very carefully when we want to improve upon it as to why it is currently not. We have seen often that optimising for just one thing is nearly always short-sighted. We optimise for human nutrients and against pathogens, with perfect hygiene, and then find that we have starved our microbiome, and that is where we got our neurotransmitters, and destroyed our immune system. We kill the large predators, and then find the forest grazed to death. We produce substances impervious to biological decay, and rejoice, and then find that we have filled the planet with trash that nothing can eat, choking everything. We isolate and overdose micronutrients to get superhealth, and find that they suddenly make us sick without the whole foods they were in. We seal our roads to be perfectly smooth, and then get flooded as the water cannot drain. We fertilise our trees for perfect growth, and do not realise we killed their fungal partners in the process and destroyed their communication system. We protect the crops with pesticides, and find that the pollinators collapse. We discover and burn fossil fuels for abundant energy, and find we have destroyed the climate.
I do think nature can, and should, be improved upon. Improvement is at the very core of evolution, nature is never done, it is ever shifting, changing, reaching. It is not per se good at any one time, just the best it could do, and this best is created out of nothing but errors, the best errors that were selected. If you will, life is not right, but it is less wrong. So I do think it contains a wealth of knowledge and experience we do not immediately see, but that is crucially important, from all the improvements that already preceded us. There are so many apparent imperfections in nature that aren’t imperfect at all, but held in the pool for the unexpected moment where we will suddenly need them again. The blood disease that makes your blood less good at transporting oxygen… but makes you resistant against a severe epidemic. The stupid appendix that can kill you when infected… but is also the safe harbor for your microbiome that will recolonise and save you if its main home is destroyed. The queer offspring that will never reproduce… but that supports, without any competition, the children of their silblings, and so brings through children at times where the restricting factor isn’t birthing, but raising. The neurodivergent children who seems oddly terrible at so many things… but then startlingly brilliant at others.
Diversity is a massive strength of nature. It is the reason we do not all fall as one, that we can survive so much. The strange can become the utterly necessary in strange times. If you encounter something awful in nature over and over, it is generally tied to something good you have not yet figured out, as a consequence or condition or correlation, or has an unexpected use that is not yet apparent, but will be crucial when it does become apparent. It can be possible to take it out, and perfect nature. I love technology that actually does, that seemlessly and gently integrates into a system and makes it more stable, more diverse, that enables self-healing, that becomes a constructive part of the whole. I love using such things, I admire them, they seem the culmination of life as an engineer of its own world, of life not being created, but the thing that creates itself and transforms the world around it. I love things that seed new opportunities, stabilise what falters, enable something novel, heal. But when we view something at a glance, and notice something that seems silly, and eradicate it… we may also remove something else that was important. I think changes need to be done with knowledge, and great care, and observation, and consequences considered. Or what we create will not be better, but instead narrow, fragile, impoverished.
“GeneSmith”… the pun just landed with me. nice.
Curated. This post is a feat of scholarship, well-written, and practical on a high impact topic. Thank you for not just doing the research, but writing it up for everyone else’s benefit too. As someone who’s personally tried for polygenic screening for IQ, etc., I wish I’d had access to this guide last year.
Sorry I couldn’t get it out earlier! I meant to release this in June of last year but the research project into which IVF clinics are best turned out to be quite a bit more difficult than I anticipated.
@GeneSmith, thanks for putting together such an interesting read! Echoing @Ben Podgursky’s comments (I’m a Genetic Counselor Orchid), we’re very proud of our monogenic screening that really goes above and beyond carrier screening that’s typically offered to IVF patients. We’re frequently asked “how often do you find something” on this screening. Given that Orchid is the first PGT lab to offer general screening for clinically significant monogenic conditions, there isn’t data available for direct comparison. Instead our detection rate estimate is based on whole genome sequencing studies in health populations and the diagnostic yield of WGS in affected individuals – the latter of which tends to have large variation across studies. To give an example, Vissers et al in 2016 reported a diagnostic yield of 60% for intellectual disability whereas Stefanski et al. in 2021 reported 28%.
Of our estimated 3.7%-3.9% detection rate of monogenic findings in embryos, we anticipate ~3% to be inherited while ~0.7-0.9% are likely to be de novo. While these may seem like small numbers, for families who have already experienced a de novo mutation, no number feels small enough and they welcome any opportunity to reduce those risks!
My partner and I put some effort into benefits from polygenic screening, but alas weren’t able to make it work.
Quick details: we had IVF embryos created and screened for a monogenic disease, (1) this didn’t leave us with enough embryos to choose anything, (2) our embryos were created and stored by UCSF clinic, and any screening would have required transferring to another clinic which would have been time consuming and expensive. Unfortunately two rounds of IVF implantation were unsuccessful, so notwithstanding the monogenic disease risk (unclear how bad it’d be), we’ll be trying the natural route.
My guess is the whole process goes better if you plan it from the start and choose a clinic accordingly, unlike us who used UCSF without realizing the impact that’d have.
Hi Ruby,
Sorry to hear your IVF process didn’t work out. UCSF was in the top 59% of clinics nationwide in 2020 and the top 38% in 2019, so while the clinic you chose may not have been the best, you at least didn’t pick a bad clinic.
Your experience is unfortunately fairly common among IVF patients. Most parents using the procedure are just hoping for at least one child through the process, and many don’t have enough embryos to even consider polygenic screening.
I really hope someone does a clinical trial of embryo splitting soon. There’s a roughly 50% chance of success using the process in animals. I bet with research we could get it up to 80-90%, which would make it viable for increasing live birth rates among parents who don’t have many embryos. That’s the type of procedure which would have improved the odds of success for parents like yourselves.
I think there is a lot of space in China for a startup to tackle a lot of these problems. What are the exact things that companies in the West don’t offer, and how difficult are they to do? I assume that all sequencing would be done locally, and the Chinese side just needs to handle data analysis Westerners are reluctant to do?
One place with a lot of low-hanging fruit is just making predictors that perform better for east asians. There are groups working on this at the moment but none of them have released high quality East Asian predictors to the public yet. Another would be offering screening for traits besides just disease. There may be some companies doing the latter in the US but they are not public at the moment.
Another really big differentiator would be if you can create better predictors for non-disease traits. There are many important non-disease traits that parents would likely want to select for, but the predictors for them are not good simply because biobanks don’t collect the needed data. You COULD start your own biobank, or perhaps if you have really good connections, get one of the Chinese biobanks to collect the data for you.
Among the traits I’m thinking of would be things like:
personality
mental energy
tolerance to sleep deprivation
pro-social tendencies
interpersonal skills
Our predictors for these traits are terrible right now.
It’s worth noting though that there is already at least one Chinese company offering polygenic embryo screening for diabetes.
If you wanted to make your own biobank it would probably cost on the order of $20-$50 mil. So pretty expensive for a startup. If you could get access to genotype and phenotype data from Chinese biobanks (or even those from Taiwan or Japan), you could probably train some pretty good predictors for maybe $100k-$200k of labor from a reasonably skilled data scientist.
But you’d then run into the problem Orchid is having right now: convincing clinicians to offer your test, or convincing patients to request it. That’s actually one of the most underrated challenges. By default, the answer from most clinics will be no. And if you’re offering a secondary service, you will need to do a lot of educational outreach because most patients and clinicians have never heard of PGT-P. And those that have are often misinformed by the cadre of academics who publish misleading studies on the subject trying to show it doesn’t work. There have been less than 500 children born via PGT-P worldwide at this point.
If you JUST wanted to offer data analysis services for western clients, your main challenge would be data access. You’ll need access to data from biobanks or from 23&Me and they are very conservative when it comes to uses for things like embryo screening.
The LessWrong Review runs every year to select the posts that have most stood the test of time. This post is not yet eligible for review, but will be at the end of 2024. The top fifty or so posts are featured prominently on the site throughout the year. Will this post make the top fifty?
Could you site the studies that this section was based on. I would be interested in reading further as this seems to be the sticking point for most people when it comes to the topic of GM for embryos.
https://www.nature.com/articles/s41598-020-69927-7
This is one of the better papers I know of examining sibling validation. To quote from the article:
There’s more in the paper if you care to take a look.
I just spoke to someone at Orchid who says their basic pricing is $2,500 per embyro for their most thorough testing, but they do have some sort of volume discount. They didn’t tell me when the discount kicks in.
Yikes. That’s even higher than I had heard.
Regarding adding IQ tests to biobank data: I doubt this will happen soon. Steve Hsu recently indicated in a podcast with Alex Murshak that he thinks it will still take many years before biobanks will have a sufficient mass of IQ tests to go along with their data. Of course, this isn’t due to a technical hurdle. (The Wonderlic, for instance, is an easy test you could administer to biobank participants at low cost.) Rather, it’s due to the fact that this research is in general verboten in the West; in China, the failure to gather IQ test data along with other data is due more to inadvertence than anything else.
That said, I do think there are some clever ways you could probably get information that is nearly as good as IQ tests. For example, working memory test batteries (consisting of complex span tasks) and processing speed test batteries seem to approximate fluid intelligence really well. Gathering this data could probably be more easily justified because it’d probably help with other kinds of neurological research (e.g., dementia research maybe?).
I have a friend who’s a geneticist and is much more pessimistic about the expected impact of embryo selection on a given desired trait. I chatted with him and read some papers he suggested and I now have a lower estimate of the expected value of embryo selection than before.
Very curious to hear from other experts in this field who disagree, especially those who don’t have a financial conflict of interest!
It seems it’s quite unclear how to even measure how heritable a given trait is, with different methods giving wildly different answers. And even if a trait is highly heritable:
The variation between siblings (or rather embryos from the same parents) is likely to be quite a lot lower than the variation seen in the general population.
The variation that’s due to very rare genetic variants is likely not captured in today’s polygenic risk scores.
Some relevant papers, which I don’t claim to fully understand, but from which I’ve extracted the above as “the gist”:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6130754/
Key thing there is probably table 2, which shows different estimates of heritability from different methods.
https://pubmed.ncbi.nlm.nih.gov/32997097/
Key quote:
“Therefore, the so-called SNV-based heritability gives the upper limit of the variance between people in their liability to a disease that can be explained by PRS and represents the variance explained by common DNA variants. As GWAS sample sizes increase, the variance explained by PRS will also increase and approach the SNV-based heritability. The SNV-based heritability estimates vary across diseases, but an approximate upper limit is approximately 30%. Although in principle, use of whole-genome sequence data could increase the variance explained by PRS (because more variance would be tagged by measured markers, ie, the SNV-based heritability approaches the heritability), it is unlikely (at least in the short term) to improve PRS (eAppendix in the Supplement). Risk stratification based on current and future PRS is illustrated in Figure 4.”
The supplement is mostly a verbal argument along the lines of “there are just huge number of rare variants, each of which exists in only a few people, so it will be difficult to figure out how to use them to improve phenotype prediction.”
Yes, I hear criticisms like those made by your friend all the time. They aren’t particularly discouraging to me.
The variation is not that much lower. The standard deviation of any continuous trait is about 70% that of the general population. That’s still plenty for selection.
The impact from rare genetic variants is mostly NOT captured in today’s genetic predictors, and that’s a place where the field could improve more in the future. But you don’t NEED rare genetic variants for embryo selection to work. You just need a predictor that correlates strongly enough with the actual trait for selection. And for many traits we already have that.
The paper you linked showing heritability is lower using relatedness disequilibrium regression was interesting. They don’t include intelligence or most of the disease predictors on which embryos are currently selected in the table, so it’s hard to give a good estimate of how strongly we should expect it to affect future increases in the efficacy of embryo selection.
If I just kind of blindly extrapolate based on the heritability of height in their study and what I know about the heritability of height vs intelligence in other studies, I conclude that the RDR method would generate an estimate of the heritability of intelligence of about 35-40%, which sounds pretty low, but not absurd.
It seems pretty likely we could still get a predictor that explained 30% of variance in the trait just using SNP arrays and large sample sizes, so my estimate of the efficacy of embryo selection for intelligence in the future doesn’t change much based on that.
This is true, but from everything I’ve read this seems to not matter that much for one simple reason: rare alleles with small effects don’t account for that much of the variance in trait values. Like if the RDR method’s estimate of heritability is accurate for height, we should expect rare variants to raise the variance explained from 45% to 55%, which would result in a ~10% increase in expected height if you were selecting embryos based on that trait.
That would be nice, but it’s not necessary for embryo selection to work.
If your friend wants to talk about this more I’d be happy to have a conversation with them.
It’s a pity they don’t use some more accurate well-being metrics like f.e. WELLBY (although I think WELLBY isn’t ideal either).
How much control do the parents have on what metric will be used to rank the embryos?
I know Genomic Prediction at least has used “Quality Adjusted Lifespan” in their past papers, so I think they’re used interchangeably.
Both they and Orchid provide the expected absolute lifetime risk of each disease in their reports, so parents can re-prioritize embryo implantation according to their preferences. Doing this methodically is pretty tricky though; every disease has a different age of onset distribution and a different impact on life expectancy and quality of life.
My hope is that one or both of them create more granular tools to help patients pick embryos according to their values and preferences. But so far the best you can do is just looking at the raw numbers and googling stuff about average age of onset etc.
Oh, so the option to choose all of those disease weights is there, it’s just a lot of effort for the parents? That’s good to know.
Yeah, ideally it shouldn’t need to be done by each parents separately, but rather there should be existing analyses ready. And even if those orgs don’t provide a satisfactory analyses themselves, they could be done independently. F.e. collaborating on that with Happier Lives Institute could work well, as they have some similar expertise.
No, the option to select against all diseases in proportion to their impact on quality-adjusted lifespan is the default. But parents can re-do the calculation to like take age of onset into account if they want. Or they could add other non-disease traits to their selection criteria (like intelligence, as estimated by some third party service).
I agree, it’s very sub-optimal for parents to have to do all this themselves.
A great overview, thanks for writing it! I think it will help a lot of people. I suggest a few corrections though:
SNP genotyping doesn’t use next generation sequencing, it uses microarrays. (Also it’s more properly called genotyping not sequencing)
I should note, in some cases embryos with aneuploid cells in the trophectoderm actually have a mixture of aneuploid and euploid cells, and the euploid cells can successfully grow into a healthy embryo. So an aneuploid trophectoderm biopsy doesn’t necessarily mean the embryo is not viable. (Although it does provide some evidence for that.)
Thanks for the correction
Sorry I may have deleted your response to my mosaicism comment when I merged it with this one. I agree that it’s controversial.
I should note, in many cases embryos with aneuploid cells in the trophectoderm actually have a mixture of aneuploid and euploid cells, and the euploid cells can successfully grow into a healthy embryo. So an aneuploid trophectoderm biopsy doesn’t necessarily mean the embryo is not viable. (Although it does provide some evidence for that.)
Yes, I thought about mentioning mosaicism but it’s such a can of worms with so much debate in the PGT research field that I just left it out.
My reading of the research suggests that mosaicism is far less common than the average PGT test would suggest. Here’s a well done study from 2021 in which the authors took apart 942 embryos and found the true rate of mosaicism to be 3%.
Most PGT labs diagnose mosaicism at far higher rates: 5-15% at most labs. My impression is this is mostly an artefact of noisy, low-density NGS sequencing technology and/or contaminated samples.
It’s also worth noting that we have a single digit number of examples of “mosaicism” in actual people, which suggests to me that there’s either differential apoptosis of aneuploid cells during embryonic development, or that mosaicism is almost always lethal.
I’ve been wanting a LW article on polygenic screening for some time now, so thank you so much for writing it. I’ll be going through it in detail later. For now, I’d just suggest rewording this:
“The average gene has a tiny, tiny impact on how long you spend in school. The predictor used 2,925 genes to explain just 15% of the variance in how many years of school a person completed.”
Instead of talking about the average gene, consider pointing out that “the most important gene” has only a tiny tiny impact on how long you spend in school. Of course, with 20-25,000 genes, the average gene was never going to be very impactful even if a single gene controlled 100% of educational attainment.
The point about 2925 genes controlling 15% of variance in years of schooling doesn’t support a candidate gene hypothesis—it supports a limited role for genes in controlling educational attainment. Even if just one gene was responsible for all of the genetic component in educational attainment, adding in the other 2924 genes would result in the same 15% of variance explained.
Thanks for the suggestion. I’ve edited the post.
It may not have saved, it still reads the same way to me (on a different device, so it’s not just cached or anything like that).
As a newbie to this intriguing topic, I have various questions:
How many genes out of 20000 commonly vary between humans?
Do more complex traits like intelligence have more gene-gene interactions?
Of the total variance, do you know what’s the maximum you could explain with genes?
Assuming the polygenic scores are not close to the maximum explainable variance: how do you know that there’s not a “complex web” on top of some additive effects? Consider the following toy model: y=β0+β1x1+β2x2+αhash(x1x2). Given data generated from it, you could infer β but the hash would fall in the error variance. Even though evolution may enforce simple effects for currently varying genes, a complex web could appear on top of fixed genes; and then I’d expect a continuum of interactivity from old, fixed genes (strong interactions, “complex web”) to new variants (additive effects).
There’s about 4-5 million letters in the genome where at least one percent of humans have a different letter at that location. That’s compared to 3 billion letters overall.
Another way to look at genetic differences is to pick a random pair of humans and ask how much they are likely to differ. The answer is by about 3 million base pairs.
That’s not my impression from reading the literature. There was some giant analysis of educational attainment done last year which found literally zero gene-gene interactions. But I’m not a deep expert on this subject.
For intelligence? You can probably get to 1/3rd of variance explained just using SNP arrays like they collect for 23&Me. With whole genome sequencing and more samples you could probably get up to 45%, maybe higher.
Gwern has written quite extensively about this.
This is not a theoretical assertion but an empirical one. We have studies on educational attainment with like 3 million participants now that have shown ZERO gene-gene interactions. They definitely exist, (at least for other traits) but according to the authors I guess you need an even larger sample size to identify them. Given how little they expect to improve the predictors power by increasing the sample size, one can infer that these interactions, if they exist (and they surely do to some extent), just don’t explain very much of the variance. (Ctrl+F for “epistatic interactions” in this paper)
Ok. I guess that, for two random humans, you expect almost all 20000 genes to differ at least on a letter, right?
Ok, but this shows that your models do not see the non-additive effects, not that there aren’t any. I don’t know exactly how analyses are done, but assuming they look at interactions with a model like y=β0+β1x1+β2x2+β12x1x2, then they would not pick up the α term in my example because of the hash (the “hash” stands for any very granular and nonlinear function).
But actually I think that it would be very weird to have such “stenographic” interactions only, without also simpler ones, so I’m satisfied with your answer.
Many of the differences between human genomes are actually in “promoter” regions. For a gene to be synthesized into a protein a little enzyme has to come over and bind to a spot next to the gene and transcribe the sequence into mRNA.
Other differences are in regions that don’t seem to affect traits at all. There’s a lot of leftover DNA in our genomes from endoviruses, transposons and other events in our evolutionary history. Sometimes the DNA in those regions randomly mutates into something useful and evolution will start acting on it.
I think there’s a good chance that transposon count in the DNA differs between people, shotgun sequencing can’t tell you how many times a given sequence appears.
There’s a tradition in biology to consider things that can’t be easily measured as irrelevant, but that doesn’t mean it’s true.
The effect of structural variants like that would be bounded by the difference between SNP heritability and full heritability. That’s an easy measurement. (And if it was really responsible for much variance, then it ought to show up as a variance component with whole-genomes from long-read sequencing, I would think.) What evidence is there that transposon counts really matter much in terms of total variance phenome-wide?
Transposons activity is not downregulated in the placenta while being downregulated in most other cells. If there are too many transposons in the DNA that likely makes the placenta fail during the pregnancy. As a result, the amount of variance in transposon counts you see when sequencing born people is more limited than that of embryos.
If you do IVF you care about the pregnancy not terminating after three or four months.
The evidence is more of a theoretical argument. In the selfish gene frame, transposons (even when they aren’t technically genes) “benefit” from copying themselves even when it reduces the total fitness of the organism.
You need a mechanism that prevents people with too many transposons from procreating to account for transposons counts not just growing indefinitely.
In the absence of a process that regulates a quantity, you have variance. Lack of observed variance in sequenced adult genomes is evidence of a mechanism for regulation existing.
To the extent that transposon sequences affect traits and risks but can’t be measured we should expect that be reflected in “missing heritability”.
You may very well be right that highly repetitive sequences like transposons do affect traits, but that’s accounted for.
EDIT: An earlier draft of the post failed to account for the cost of medications in the price of IVF and subsequently understated the expected cost of IVF by $4000. The costs table has been updated to reflect the changes.
Given that this is still fairly underground with all the secret startups, contact Jonathan etc, how should this impact on deciding to do this sooner rather than later? From reading this, it seems like this is not particularly difficult to do in 2023, providing you know where to look, and will likely get easier in the next few years.
My concern is that as this becomes more widespread, sociopolitical pushback will cause it to become more difficult/costly/less effective, either through social pressure or regulation. You have mentioned already that companies have removed certain predictors; I suspect that this is just the beginning, and more obstacles will appear as this becomes more widely known among the public.
From this perspective, I would argue that people should plan to do this in the next few years, while it is comparatively unknown/unregulated.
I actually see things going the other way: about 30% of Americans already support gene editing for intelligence enhancement, and almost 50% support embyro selection. My prediction is this tech will gain widespread acceptance.
IVF was pretty unpopular when it first came out, but over time as people came to see that the children born via the process were normal, it became much more acceptable to the point where nowadays it’s kind of weird to view IVF as immoral.
Embryo selection for health, intelligence and happiness is completely consistent with our existing values. And it’s not scary in the way eugenics is because you don’t need top-down government control of reproduction for it to work.
If we just ignore AI for the moment (or assume progress slows down a lot due to regulations/restrictions), I predict the fight is going to shift towards cost and access. People are going to be upset that rich people are securing an advantage for their children that they can’t afford.
But regardless of whether it becomes popular or causes a backlash, it makes sense to freeze embryos NOW. Female age is the single biggest predictor of gain for a fixed price, so the sooner you go through the process the better.
I think you should mention that traits tend to be correlated. Genomic Prediction has a company policy of not telling you the polygenic scores for height, education attainment, or even cosmetic traits like the eye color of the embryo (this last one can be nearly 100% accurate). Understandably, they don’t want to be accused of being an eugenics shop. However, there’s strong correlation between height, education attainment, and lower mental disease risk. If you optimize for lower disease risk and height, you will get high education attainment as a by product.
Explicitly screening for IQ is what we want. I don’t know when this will happen without all the associated baggage from people who won’t even accept the very concept of behavior genetics.
Hm. I wonder if there are people who simultaneously say that genes don’t affect behavior, and also say that genetic screening is bad. Seems like a near-contradiction, but I suspect there are such people.
There seem to be some disease genes correlated with higher IQ. There’s speculation about whether genetic conditions in Ashkenazi Jews cause higher intelligence, but there’s also a gene that causes blindness in middle age that also appears to raise intelligence by enhancing neuronal signaling.
In general, selective breeding of animals for various traits have often managed to produce animals that excel in that trait but are noticeably less healthy overall. At this point, I don’t think actually know which genes are tradeoffs and which are just flaws—including genes that trade off against something that doesn’t matter as much in the modern world, such as resistance to famine conditions or infections treatable by antibiotics. We certainly can try to weigh the odds and try to avoid the cases where things have Obviously Gone Wrong, but I reserve the right to worry about removing genes from the human gene pool whose function we don’t understand.
It’s very reasonable to worry about these things, but let me give you a couple of reasons why I am not very concerned about negative plieotropy:
We can observe in actual real humans right now that there is a negative correlation between IQ and disease risk. As you have pointed out, there are some exceptions to this such as autism risk. But the general picture is pretty optimistic.
Imagine for a moment that we have two sets of alleles: one set increases IQ. The other set increases disease risk. The sets are not disjoint; that is to say there are some alleles which both increase IQ AND disease risk. If we simply select for IQ, we will select both from the set of genes that ONLY increase IQ with no effect on disease, AND for the set of genes that both increase IQ and disease risk.
If you add lower disease risk to your selection index, you will disproportionately select for alleles that are in the subset of variants that increase IQ without increasing disease risk. You’ll also select for some alleles that have no effect on IQ but decrease disease risk.
Now you might be worried that perhaps there’s some disease that isn’t in our selection index but nonetheless has variants which increase both risk and IQ.
Fortunately, there’s a way to deal with this too: add traits that are affected by pretty much all diseases to your selection criteria: for example:
Of cou
Likelihood of developing chronic pain
Self-rated quality of life
Life expectancy
The two companies that currently offer polygenic embryo screening are already selecting against or for some of these to a limited degree by using disease risk as a proxy. But they could and should select on them directly to deal with the problem you described.
So I agree that if you select very very hard on a small set of traits, you are likely to get unforeseen downsides somewhere down the road. But two things worth considering here:
Some of the health problems in domesticated animals related to inbreeding rather than selection on a variety of traits.
Health was basically not used as a selective trait for livestock except insofar as it affected the traits that people actually cared about (namely meat, egg and milk production)
Domesticated animals have been pushed like a dozen or more standard deviations from their pre-selection means for many traits. We don’t have the selection power to do that with embryo selection except on a time scale of hundreds of years. It’s another story with like CRISPR editing or iterated embryo selection, so I do think we’ll have to be much more cognisant of that issue down the road.
We are not going to be removing any of these genes from the gene pool for a long time. It’s going to take 20-50 years for this technology to become widely adopted around the world. And even then, you’re unlikely to get the elimination of any genes without editing or iterated selection of some type.
I actually do agree with you here. At least in the short run, human knowledge probably already is better than past natural selection at picking genes that result in humans that end up healthy, intelligent, and fertile in the modern world that includes C-sections, baby formula, abundant calories, and antibiotics.
My wife’s family in particular tends to be very unhealthy and die young. She’s 31 now and if she manages to recover from her life-threatening medical conditions to the point that she can safely carry a pregnancy to term, it would help very much if we could do something to reduce our future child’s risk of obesity, type 2 diabetes, kidney disease, and heart disease so they won’t die before the age of 50.
I’m sorry to hear about your wife’s condition. I’ve had family members that have died young from diseases that most people get in old age as well. It’s a very painful experience.
If your outlook improves and you decide to do polygenic embryo screening, reach out to me and I can help give you a more personalized assessment of your prospects. The size of the absolute risk reduction you can get from embryo selection will actually be higher if your or your wife’s risk is higher. So the benefit will likely be higher for the two of you.
Also, the predictors for type 2, obesity, and heart disease are already very good, so if those are your concerns you’re in luck.
You’re right. I’ll edit the original post. I originally didn’t include it because i couldn’t quantify it that well, but it’s worth at least mentioning.
Again, if you read the post I believe there are probably groups doing this right now. If you’re interested in using the service right now you can talk to Jonathan Anomaly as he seems to know how to get in contact with them.
It’s probably morally imperative that all parents who have the financial means to do this do it.
I think it’s morally important that we make this choice increasingly accessible, and fight any bigotry against children born with this method and bigotry against their parents. It would take a pretty niche moral stance and cost benefit analysis to make this morally imperative.
I suspect bigotry against children born this way would not work, just because they would be impossible to identify. (Presumably most of them would not even know themselves).
Although a future world where someone says: “phwa! You are only smarter and hotter because of gross polygenic screening your parents cheated into you.” Reply: “But, I am a lonely child selected from 4 embryos, you have 4 less successful siblings, so you are more selected than I am.”
Yes, I might write later about how to make this cheaper. Though what I would write may have limited relevance, as I expect gene editing methods to surpass simple embryo selection within the next ten years.
I’m not quite sure I would agree with this yet, though I can see the case being made for it.
I think it mostly comes down to how much you think you can improve worldwide outcomes by increasing the abilities of those at the top vs bringing up those with the least.
Iodine supplementation in the developing world, for example, is probably the single most cost-effective way of increasing average IQ per capita worldwide. It also helps prevent other problems like hypothyroidism.
So if just increasing IQ per capita is your goal, polygenic embryo selection is not going to come anywhere close to iodine supplementation.
Of course, iodine supplementation is not going to give you any more geniuses, and geniuses per capita has an incredibly strong impact on human progress.
I really, really wish we could just ban AI improvements and focus on enhancing human intelligence and morality for a few decades. The reason I originally became interested in embryo selection was that I thought that genetic engineering might be a potential solution to the alignment problem (not to mention many of the other problems the human species faces). But it’s going to take at least 20 years to work (and realistically more like 30-40) to have a large impact. I’d put the odds of us getting to AGI before that at like 90%. The only path I can see now involves a worldwide ban on AI capabilities improvements.
Suppose a family values the positive effects that screening would have on their child at $30,000, but in their area, it would cost them $50,000. Them paying for it anyway would be like “donating” $20,000 towards the moral imperative that you propose. But would that really be the best counterfactual use of the money? E.g. donating it instead to the Against Malaria Foundation would save 4-5 lives in expectation.[1] Maybe it would be worth it at $10,000? $5,000?
Although, this doesn’t take into account the idea that an additional person doing polygenic screening would increase its acceptance in the public, incentivizing companies to innovate and drive the price down. So maybe the knock-on effects would make it worth it.
Okay, I’ve heard that this scale of donations to short-termist charities is actually a lot more complicated than that, but this is just an example.
I mostly agree with this perspective with regards to the “moral imperative”.
But apart from that, it seems to me that a good case can be made if we use personal health spending as a reference class.
Even if we only consider currently achievable DALY gains, it is quite notable that we have a method to gain several healthy life-years for a price of maybe $20,000/healthy year (and actually these gains should even be heritable themselves!).
I do not know the numbers for common health interventions, but this should already be somewhat comparable.
update: Quick estimate: US per capita health spending in 2019 was $11,582 according to CDC. If the US health spending doubles life expectancy compared to having no health system, this is comparable to $20,000/healthy year.
This may be an overstatement. I think the moral minimum looks more like “bring children into the world in a way that’s consistent with the value system you plan to teach them and hope that they live by”.
If you want to teach a value system of global optimization, where every dollar should be spent to have the maximum possible impact to global quality of life, you’re probably adopting rather than conceiving anyways… but this great an investment into a single individual is likely inconsistent with those values.
If you want to teach a value system of local optimization, where every person ought to first do what’s best for themself and their loved ones before attempting to intervene in the lives of strangers, then it might be inconsistent to gamble with a family member’s lifetime wellbeing when you could instead have stacked the odds in their favor.
I think this is incorrect. Behaviors and talents are to a large degree heritable. If you want future people to share your values, one of the best ways to do that is to have kids who are disproportionately likely to share those values.
And while you can of course attempt to teach your kids your values, genetics plays a major role in determining what kinds of values we adopt.
This is one of the major reasons why “not having kids because of climate change” is not just ineffective but actively counterproductive; it ensures there are less people in the future that will be willing to make large sacrifices for the good of the whole.
I agree that many behaviors are heritable, but I model that inheritance as emerging from the intersection of genetic and environmental factors. I hadn’t previously considered generalizing from genetic behavioral proclivities to what values people hold.
Could you point me toward the data from which you’ve drawn this conclusion? I imagine that there are enough adoptee studies in the world to point at a link pretty conclusively if one exists, but I’d also like to skip straight to the most applicable ones if you could recommend them.
Political attitudes seem to be about 30-70% heritable. Interestingly, people’s genetics seem to have a stronger effect on their attitudes the more politically engaged they are.
https://www.nytimes.com/2022/06/01/opinion/politics-genetics-research.html
The improvements in the mental and physical well-being of the poorest of us if this huge sum of money was instead used to give them healthcare, safety from preventable, transmissible diseases (like malaria nets), clean water, healthy food, crucial supplements, reproductive rights, and most of all education, vastly, vastly outweighs raises the IQs of rich children by this little.
“Rich people” already give the lions share to anti malaria charities, just as virtually all of Earth’s economic surplus (for now, pre-AGI) comes from fairly-high-IQ people doing functionally prosocial things. The question is not “is standard EA behavior better than good embryo selection”—effective altruism exists because there are enough altruistic intelligent people around to be EAs—but how good existing methods are, and what runway we have to use them.
I think this is probably true at the moment given the current efficacy and the prices of IVF.
The only reason I hesitate is because I think the tendencies and aptitudes of people at the top have a gigantic impact on the rest of society and that improving those tendencies would have a very large impact on the world (especially if parents of children likely to end up in positions of power select for traits like kindness and pro-social tendencies).
But if you ignore that for a moment, then you’re of course correct that embryo selection can come nowhere close to the efficacy of distributing bed nets or supplementing iodine-deficient populations.
However, it’s going to take some time to bring down the price of embryo selection and future technologies for genetic improvement, so I think it’s probably good to start on that now.
Granted, this is all kind of ignoring the 800 pound gorilla in the room which is AI. For embryo selection to really matter, there needs to be time for these children to grow up. If we make ASI in the next 20 years, there’s probably not much point. I’m still hopeful that we can get a pause in place on AI development. But without one this whole endeavor has pretty low odds of achieving anything IMO.
“Moral imperatives” is not a category that relies upon financial means. Moral imperatives in traditional Kantian framework are supposed to be universal, no? Just because some action could be personally and socially very beneficial doesn’t make it morally compulsory. The benefits would have to be weighed against opportunity cost, uncertainty, game theoretic considerations, and possible contrary moral systems being correct.
Also, please keep the hell out of the reproductive rights of other people. At the point where you prescribe this (and according to whose ideal standards, exactly?), I genuinely no longer understand the difference that is being claimed to outright eugenics. How long until it is de facto mandatory?
Well this certainly opens a window I hadn’t really thought about before. Very intriguing stuff (have only had time to read over the “Concrete Advice” section, but it was a great overview). Not sure I would totally utilize this at the moment (the cost being a major factor), but I have no doubt this kind of thing will get more prevalent and less expensive as time goes on.
I’m wondering if others are having a bit of cognitive dissonance like myself with the taint of eugenics lingering in the air? This quote
definitely reminded me of some of the complications of this kind of thing in practice...
Yes, I agree that cost and disparities in predictor strength between genetic ancestry groups are probably the biggest issues with polygenic screening at the moment. I didn’t mention this in the post, but the issue with disease predictor disparity should improve significantly in the next year or two. There are two large biobanks coming online with significant numbers of non-European participants; The Taiwan Biobank and the “All of Us” biobank in the USA. These should at least reduce the disparity in disease predictor strength, though it remains to be seen whether either one will actually collect data on cognitive phenotypes.
I think we need a new term to describe “genetic improvement” that includes embryo selection and people choosing who to reproduce with but not state-sponsored sterilizations or genocide. The fact we use one word to describe both of those is crazy. It’s like using “stuff” to refer to both food and excrement.
“Oh yeah the stuff I got at the restaurant was good, but stuff isn’t always good so eating there made me a bit nervous”
Aella girl has suggested the term “epilogenics”. “epilogi” is the greek word for “choice”, so we can use that instead of “eu” which means “good”. I quite like this term and will be using it from now on.
So yes, I strongly oppose many types of eugenics but I am strongly in favor of epilogenics.
Yeah I tried to invoke the notion I had that there was this almost ephemeral, tenuous connection between this “stuff” and eugenics. Not trying to imply a direct line of similarity by any means. And I agree that new terminology is needed to distinguish and make useful which aspects society is willing and able to allow vs. not.
I’m glad more biobanks are coming online! I could imagine this increasing by an OOM over the next 5-10 years?
Do you have a source for the misconception being common?
No. This is drawn from my own personal experience reading comments by otherwise knowledgeable academics or professionals in the ART industry. It’s also something I read online a lot in forums such as reddit. Inevitably one of the top comments on most articles about human genetic engineering is along the lines of “we don’t understand anywhere close to enough about genetics to make the proposed changes”.
If this is really important to you I can probably find some examples.
patently false.
Neither of Einstein’s parents were Nobel laureates;
his father was a salesman, his mother was a pianist (among other talents).
This is not meant as a literal statement. I’m just trying to convey that the magnitude of benefits is still rather small.
There are obviously many examples of geniuses who didn’t come from unusually impressive families.
Shouldn’t this be triplet birthrates? Twin birthrates look pretty stable in comparison.
Do you mean “natural pregnancy?” Aneuploidy generally results in miscarriage, which we don’t call a “natural birth.”
Yes, thanks for the correction
Thanks for this awesome post. Biggest update for me is “there might be a way to get screening for traits not advertised by Genomic Prediction”, but I still have no idea of the cost or the probability of success :-) Would be great to hear from people who have more info on this.
I recently weighed the pros and cons of IVF vs old-fashioned conception and went old-fashioned because:
1. This article claims “different embryo culture media give rise to different birthweights and growth patterns in children” and “children born after ART have altered epigenetic profiles”. I’m not an expert but I read it and found it quite plausible that there are ways that IVF can cause worse health outcomes. Very hard to tell without randomized trials, and all trials on IVF vs non-IVF are going to be heavily confounded.
2. My child would be only 1⁄4 European ancestry and as you note the current predictors perform worse on non-Europeans. If we had good predictors for my child’s ancestry mix, then I’d probably go with IVF despite the possible downsides I noted above. Hopefully the new bio-banks you cited will enable that soon.
I wrote the section on cost to give you a better idea of the prices involved. Hopefully that’s helpful.
But I take your point that what is really needed is a “calculator” of some sort where you can input relevant variables and see your expected gains and costs. I am working on something like this at the moment but it may be several months until it’s finished.
Apart from the randomized control trial looking at different embryo media, I find all the studies presented in this paper to be highly suspect. For example they cite showing that fresh embryo transfer is associate with preterm birth. But the study THEY cite doesn’t even control for the differences in maternal age between parents that do IVF and those that don’t!
And surprise surprise, there is a major difference in PTB rates between women in their late 20s and early 30s and those in their late 30s to early 40s.
Perhaps I am wrong about this, but my best guess right now is that the downsides of doing IVF are very minor and are massively outweighed by the upsides of embryo selection. The cost is still a big barrier, so I can understand if you don’t do it for that reason.
Yes, this is still a problem. For IQ gain in particular though the difference is not that big. I believe east and south asians, for example, have an expected IQ gain of about 75% that of Europeans (so like 3.5-4 points vs 5). Maybe that’s a big enough difference for it to not be worth it, but it’s not a huge reduction.
One thing I didn’t see on perusal was a reference to the length of time some of these paths might take.
I know this could vary extremely according to each person’s desired timeline w/r/t keeping embryos frozen and determining the right time for implantation, etc.
But from a naive standpoint, saying that someone wanted to have children quite soon otherwise, how long might one expect to spend going through the “average” or “optimal” path doing this?
There’s generally a waiting time of 1-3 months for initial consultations, and another 1-2 months of checkups, hormone treatments and transfers. So 2-5 months before you can actually transfer an embryo, and another 9 months until birth.
Thank you!
Hi, you suggested freezing embryos instead of eggs. However, a recent study found that frozen embryo babies are 2.5 times more likely to develop cancer. At the same time, a review article on IVF comes to the following conclusion (likely due to epigenetic factors):
This makes me want to consider two points:
Assisted reproductive technology carries risks and couples that go through IVF should in any case do polygenic testing to offset these
Is the risk/benefit ratio worth it for couples that can conceive naturally, especially for something like merely 5 IQ points?
Thank you for leaving such a thought-provoking comment. I’ve spent a couple hours reading through the study you posted tonight as well as others linked to by the authors.
I don’t see the claim about a 2.5x increased risk of cancer anywhere though. From the findings section:
So the risk of cancer was 8% higher in those born after ART, and 59% higher for frozen embryos vs fresh embryos.
I think the generally higher disease prevalence among IVF couples probably explains the 8% increase for ART in general, though the 59% increase they see for frozen embryo transfer is surprising.
Looking more into the study it looks like about a quarter of the effect is driven by the higher rates of twin births in IVF, which are much less common nowadays.
This study uses data that is also quite old; they include cycles going all the way back to 1984 or 1994 for some countries. The rate of embryo freezing at that time were quite low, as evidence by the huge difference between hazard ratios for all ART and frozen embryo transfer. If frozen embryos made up a higher proportion of the births you would see a smaller difference between all ART relative to spontaneous conception and frozen embryo transfer relative to spontaneous conception.
Here’s another study that found higher risk of neoplasms among embryos that were transferred fresh. Granted, this was a smaller study, so I’d lean towards believing your study.
There’s also a graph in the study which seems to show the relative cancer risk for frozen embryos declining over time:
Though this could just reflect fewer twin births. And the confidence interals are such that it’s hard to be certain the effect is real.
Another possible confounder here is maternal age. The average age of mothers in ART were older than those in the spontaneous conception group by about 4 years. You can see in this study that maternal age is significantly associated with higher childhood cancer risk. So this could explain another 5-10%, particularly if the age of mothers in the frozen embryo group was higher (I didn’t see a comparison in the study but I could have missed it).
Still, there remains a chance that freezing embryos does increase childhood cancer risk. The absolute risk increase is still quite small: about 0.2% up to age 18. Though I suppose the increase in risk for adults could be higher?
Overall I’m just not sure what to think. This is one study and my experience with these “association studies” is they never control for confounders. Like what are the indications for embryo freezing and could those potentially account for this difference? Was childhood cancer risk the only thing the authors tested for, or are there other associations they didn’t report? I guess I’ll update my priors in favor of frozen embryo transfer increasing childhood cancer risk a bit?
Yes, this one seems like a no-brainer to me, regardless of whether or not the effects we see are caused by IVF or merely associated with it by virtue of the parents that need it having higher polygenic risk scores for disease. The fact that PGT-P isn’t already universal in IVF is kind of a tragedy. You can avoid like $200k in future medical expenses for like $3-5k. Not to mention the improvement in quality of life that comes with lower risk of obesity, clinical depression, type 1 diabetes and other early onset conditions.
If the effect is indeed real, I would happily take a .2% increase in childhood cancer risk for +5 IQ points. I don’t think that level of increased risk is really much of an issue for most parents. The much bigger issue is the cost of doing IVF. At $30k minimum, this is an expensive procedure.
I’m relatively confident there are at least a few tens of thousands of parents in the US who would do IVF for the purposes of these benefits if they knew this was possible. But for the time being with the benefits being relatively minor, the vast majority of parents will of course opt for natural birth.
With a better predictor capable of +5-13 IQ points I think that number will expand significantly. And if we can lower the cost of IVF I expect it to expand much farther. But we have to start somewhere.
I think Widen et al. (2022) uses actual sibling pairs/trios (unless I’m misreading?), but a few other studies use simulated embryos such as Lencz et al. (2021) [1] and Turley et al. (2021) [2].
[1] “Utility of polygenic embryo screening for disease depends on the selection strategy”
[2] “Problems with Using Polygenic Scores to Select Embryos”
If you look at the discussion section they say “Specifically, we validated this index in selection experiments using unrelated individuals and sibling pairs and trios from the UK Biobank.”
The graph of relative risk reduction I placed in the post shows reductions among groups of five. They state elsewhere in the paper that there were 969 trios available in UK Biobank, the source of data for the paper. There is simply no way they could have produced confidence intervals as tight as those shown in the graph from real families of five siblings. I would be surprised if there were more than 100 such families in all of UKBB.
Hence I concluded they must be using pseudosiblings.
My estimates for the expected reduction we’d see moving from pseudosiblings to real siblings are pretty rough, but it’s based on Figure 7 in the paper where they show the relative risk reduction size between siblings and unrelated individuals. There’s a lot of variance in the chart, but it looks like there’s maybe a roughly 20% reduction in RRR when moving from pseudosiblings to siblings.
My guess is that intelligence is anti-correlated with happiness. Now, following the Achmiz’s law,
A Non-trivial Assertion without Examples Raises Specter of Said Achmiz Demanding Infinitely Many of Them
I point at dumb dogs, and that people with below-average IQ seem happier on average (study: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5486156/ )
Thus it is unethical to knowingly select for higher intelligence, as you create unhappier offspring in expectation. It may benefit the society as a whole, and the offspring in question probably would not want to have been born “less intelligent”, but it does not change the argument.
So I agree with your general point that it is important to consider negative pleiotropy between traits. However in the specific case of happiness and intelligence, the first two studies I found from googling suggest that happiness and intelligence are positively correlated.12
Here’s a meta-analysis of 23 studies that found no correlation between intelligence and happiness at an individual level but a strong correlation at the country level.
So I think that unless you’re dealing with much stronger techniques than simple embryo selection, this is not a concern. However, if it was you could simply select for both genetic predisposition to happiness and genetic predisposition to high intelligence.
Well, I think we are in agreement, and it all comes down to evaluating expected happiness. Maybe one can select for both intelligence and happiness, But that does not seem to be covered in OP, which seems like a pretty big omission, just assuming that intelligence is an unquestionable positive on a personal scale.
Wait, it seems like those last two points would totally change the argument! Consider:
“It is unethical to donate to effective-altruist charities, since giving away money will mean that your life becomes less happy. It may benefit society as a whole and lead to greater happiness overall. But it does not change the argument: donations are unethical because the donation makes your own life worse.” This seems crazy to me?? If anything it seems like many would consider it unethical to keep the money for yourself.
Your logic would seem to go beyond “don’t use embryo selection to boost IQ, have kids the regular way instead”. It seems to extend all the way to “you should use embryo selection to deliberately hamstring IQ, in the hopes of birthing a smiling idiot”. Am I thus obligated to try and damage my child’s intelligence? (Perhaps for instance by binge-drinking during pregnancy, if I can’t afford IVF?)
It also seems like the child’s preferences would matter to this situation. For instance, personally, I am a reasonably happy guy; I wouldn’t mind sacrificing some of my personal life happiness in order to become more intelligent. (Actually, since I also consider myself a reasonably smart guy, what I would really like is to sacrifice some happiness in order to become more hardworking / conscientious / ambitious. A little more of a “Type-A” high-achieving neurotic… not too much, of course, but just a little in that direction. I think this would improve my material circumstances since I’d work harder, and it would also be better for the world since I’d be producing more societal value. Having a slightly more harried and tumultuous inner life seems like an acceptable price to pay; I know lots of people who are more Type-A than I am, and they seem alright.) I would hate for someone to paternalistically say to me: “Nope, you would be happier if you were even more of a lazy slacker, and had fewer IQ points. So you’re not allowed to trade away any happiness. In fact, I’m gonna turn these intelligence and conscientiousness dials down a few notches, for your own good!”
I guess this is just the classic conflict between preference utilitarianism vs hedonic utilitarianism. But in this situation, preference utilitarianism seems (to me) to be viscerally in the right, while hedonic utilitarianism seems to be doing something extremely cruel and confining.
To be clear, I also dispute the idea that more intelligence --> less happiness. You could probably find some narrowly-defined type of happiness which is anticorrelated with intelligence. But a lot of the meaning and happiness in my life seem like they would get better with more intelligence. Like my ability to understand my place in the world and live an independent life, planning my career/relationships/etc with lots of personal agency. Or my ability to appreciate the texture/experience of being alive—noticing sensations, taking time to “smell the roses”, and making meditative/spiritual/introspective progress of understanding my own mind. My ability to overcome emotional difficulties/setbacks by “working through them” and communicating well with the person I might be angry at. My material quality of life, enabled by my high-income job, which I couldn’t hold down if I wasn’t reasonably smart. My ability to appreciate art on a deep level (see my lecture series about the videogame “The Witness”, an intellectual pursuit which brings me great joy). And so forth.
A few points:
Oh come on, this is an informed personal choice, not something your parents decided for you, why would you even put the two together.
I said or implied nothing of the sort! Maybe you can select for both intelligence and emotional stability, I don’t know. Just don’t focus on one trait and assume it is an indisputable good.
Yes, so would I! Again, when it is a personal informed choice, the situation is entirely different.
May well be, I linked a study to that effect, it might be wrong, or not replicated. But you don’t get to discard evidence just because you do not like it.
Thanks for all these clarifications; sorry if I came off as too harsh.
“Yes, so would I! Again, when it is a personal informed choice, the situation is entirely different.” -- It seems to me like in the case of the child (who, having not been born yet, cannot decide either way), the best we can do is guess what their personal informed choice would be. To me it seems likely that the child might choose to trade off a bit of happiness in order to boost other stats (relative to my level of happiness and other stats, and depending of course on how much that lost happiness is buying). After all, that’s what I’d choose, and the child will share half my genes! To me, the fact that it’s not a personal choice is unfortunate, and I take your point—forcing /some random other person/ to donate to EA charities would seem unacceptably coercive. (Although I do support the idea of a government funded by taxes.) But since the child isn’t yet born, the situation is intermediate between “informed personal choice” vs coercing a random guy. In this intermediate situation, I think choosing based on my best guess of the unborn child’s future preferences is the best option. Especially since it’s unclear what the “default” choice should be—selecting for IQ, selecting against IQ, or leaving IQ alone (and going with whatever level of IQ and happiness is implied by the genes of me and my partner), all seem like they have an equal claim to being the default. Unless I thought that my current genes were shaped by evolution to be at the optimal tradeoff point already, which (considering how much natural variation there is among people, and the fact that evolution’s values are not my values) seems unlikely to me.
Agreed that it is possible that IQ --> less happiness, for most people / on average, even though that strikes me as unlikely. It would be great to see more research that tries to look at this more closely and in various ways.
And totally agreed that this would be a tough tradeoff to make either way; that selecting for emotional stability and happiness alongside IQ would be a high priority if I was doing this myself.
I agree with all these considerations and the choice not being straightforward. It gets even more complicated when one goes deeper into the weeds of the J.S. Mill’s version of utilitarianism. I guess my original point expressed less radically is that assuming that higher IQ is automatically better is far from obvious.