So What’s Up With PUFAs Chemically?
This is exploratory investigation of a new-ish hypothesis, it is not intended to be a comprehensive review of the field or even a a full investigation of the hypothesis.
I’ve always been skeptical of the seed-oil theory of obesity. Perhaps this is bad rationality on my part, but I’ve tended to retreat to the sniff test on issues as charged and confusing as diet. My response to the general seed-oil theory was basically “Really? Seeds and nuts? The things you just find growing on plants, and that our ancestors surely ate loads of?”
But a twitter thread recently made me take another look at it, and since I have a lot of chemistry experience I thought I’d take a look.
The PUFA Breakdown Theory
It goes like this:
PUFAs from nuts and seeds are fine. Deep-frying using PUFAs causes them to break down in a way other fatty acids do not, and these breakdown products are the problem.
Most of a fatty acid is the “tail”. This consists of hydrogen atoms decorating a backbone of carbon atoms. Each carbon atom can make up to four bonds, of which two have to be to other carbons (except the end carbon which only bonds to one carbon) leaving space for two hydrogens. When a chain has the maximum number of hydrogen atoms, we say it’s “saturated”. These tails have the general formula :
For a carbon which is saturated (i.e. has four single bonds) the bonds are arranged like the corners of a tetrahedron, and rotation around single bonds is permitted, meaning the overall assembly is like a floppy chain.
Instead, we can have two adjacent carbons form a double bond, each forming one bond to hydrogen, two bonds to the adjacent carbon, and one to a different carbon:
Unlike single bonds, double bonds are rigid, and if a carbon atom has a double bond, the three remaining bonds fall in a plane. This means there are two ways in which the rest of the chain can be laid out. If the carbons form a zig-zag S shape, this is a trans double bond. If they form a curved C shape, we have a cis double bond.
The health dangers of trans-fatty acids have been known for a long while. They don’t occur in nature (which is probably why they’re so bad for us). Cis-fatty acids are very common though, especially in vegetable and, yes, seed oils.
Of course there’s no reason why we should stop at one double bond, we can just as easily have multiple. This gets us to the name poly-unsaturated fatty acids (PUFAs). I’ll compare stearic acid (SA) oleic acid (OA) and linoleic acid (LA) for clarity:
Linoleic acid is the one that seed oil enthusiasts are most interested in. We can go even further and look at -linoleic acid, which has even more double bonds, but I think LA makes the point just fine.
Three fatty acids, usually identical ones, attach to one glycerol molecule to form a triglyceride.
Isomerization
As I mentioned earlier, double bonds are rigid, so if you have a cis double bond, it stays that way. Mostly. In chemistry a reaction is never impossible, the components are just insufficiently hot. If we heat up a cis-fatty acid to a sufficient temperature, the molecules will be able to access enough energy to flip. The rate of reactions generally scales with temperature according to the Arrhenius equation:
Where is a general constant determining the speed, is the “activation energy” of the reaction, is temperature, and is a Boltzmann’s constant which makes the units work out. Graphing this gives the following shape:
Suffice to say this means that reaction speed can grow very rapidly with temperature at the “right” point on this graph.
Why is this important? Well, trans-fatty acids are slightly lower energy than cis ones, so at a high enough temperature, we can see cis to trans isomerization, turning OA or LA into something you 100% definitely do not want to be eating. As far as I’m aware nobody claims trans fats aren’t bad.
So what’s the right temperature, if it’s then we don’t care. The literature seems to be somewhat split on how fast this reaction is. One study claims that after 5 hours, 0.2% of LA is converted to a trans form at 140 , and about 1% is converted at 220 (284 and 428 F respectively).[1] Another finds that after 1 hour, trans isomers are negligible at temperatures below 220 .[2]
This second study also notes that OA is converted to trans isomers at a higher rate than LA which is corroborated by a study of OA breakdown.[3]
Now lets think about how much trans fat is likely to be produced during a typical deep frying episode. At-home deep frying typically lasts <1 hour in my experience (usually less than 30 minutes), although oil might be re-used several times, which is probably something to consider.
McDonald’s on the other hand… changes their frying oil every two weeks. 8 hours by 14 days = 112 hours of operations. I actually can’t get great data on their frying temperature, but let’s try the full gamut of options. Here’s the graph from the paper earlier:
If we assume a conservative 160 C:
\(\ln k = −11700 \times \frac 1{433} + 20.7 = −6.3\\)
Which gives around 0.2% trans OA.
If instead they do a average-ish 175 C:
\(\ln k = −11700 \times \frac 1{449} + 20.7 = −5.5\\)
Which gives around 0.46% trans OA
Or a high temperature 190 C:
\(\ln k = −11700 \times \frac 1{463} + 20.7 = −4.6\\)
Which gives 1.1% trans OA.
I don’t know how much trans OA is harmful. Margarine is roughly 5-10% trans fats and that’s supposed to be pretty bad. It is also plausible I got these numbers wrong, since the paper insists on insane units like (g/100g) and doesn’t give any units in their table there.
The fact that OA makes trans fats faster than LA surprised me until I noticed the reason. LA is more likely than OA to be oxidized to hydroperoxides. The first study also notes up to a ~25% decrease in total un-oxidized LA after 5 hours at 220 C (I’ve said “up to” since there could be other issues at play like hydrolysis creating free fatty acids).
Hydroperoxides
As well as flipping round, double bonds can react with oxygen at an adjacent carbon to form a “hydroperoxide”. The rate of hydroperoxide formation is pretty high (again, up to 25% of the LA after 5 hours at 220 ) so for PUFAs, this is the major degradation product over normal trans-fatty acids.

“Singlet oxygen” means an excited state of oxygen which is formed at high temperatures (oxygen actually has this weird quirk which makes it unreactive, which is why we don’t normally catch fire spontaneously, try walking around in 20% fluorine or chlorine gas to appreciate the difference). Deep-frying can raise oxygen to this state, allowing it to oxidize lipids to hydroperoxides, which happens to the exclusion of the formation of trans-LA.
The hydroperoxides also often take the form trans-fatty acids as well as being oxidized. These don’t show up when looking for normal trans-LA but they’re presumably just as harmful.
Once a hydroperoxide has formed, it can catalyse the formation of more hydroperoxides, which is probably why LA (2 double bonds per fatty acid, so 6 per triglyceride molecule) is so eager. LA also has an especially vulnerable-looking carbon between two sets of double bonds.
So what might be the effects of peroxides in diet? The first paper I found contains the phrase “while fish oil had been considered poisonous, in fact, fresh fish oil itself was not poisonous, unlike oxidized fish oil”.[4] So presumably at high enough concentrations they are bad! This paper shows that oxidized oils are pretty bad, say it with me now, IN MICE and also IN HUMAN CELLS IN A DISH, but doesn’t actually give much causal evidence in humans beyond association studies of oxides in serum. Old and sick people have different stuff in their blood, more at 11.
Another paper suggests that dietary lipid peroxides might cause cancer, but doesn’t say much about the sorts of things that seed oil proponents often mention (weight gain, inflammation, etc.)[5]
Nonetheless, if these things are poisonous at high concentrations, they’re probably not great at low concentrations.
Final Thoughts
All of the degradation studies have been done on pure oils, and most likely they were done in an inert (glass) vessel. Many, many things can catalyse oxidation and isomerization reactions, including metal ions, so I suspect cooking with actual food in an actual deep fryer would cause more of both reactions.
Ideally I’d like someone to just sample the McDonald’s cooking oil every few days through a few cycles of oil changes, but I doubt this will happen.
Here’s a chart of fatty acid content:
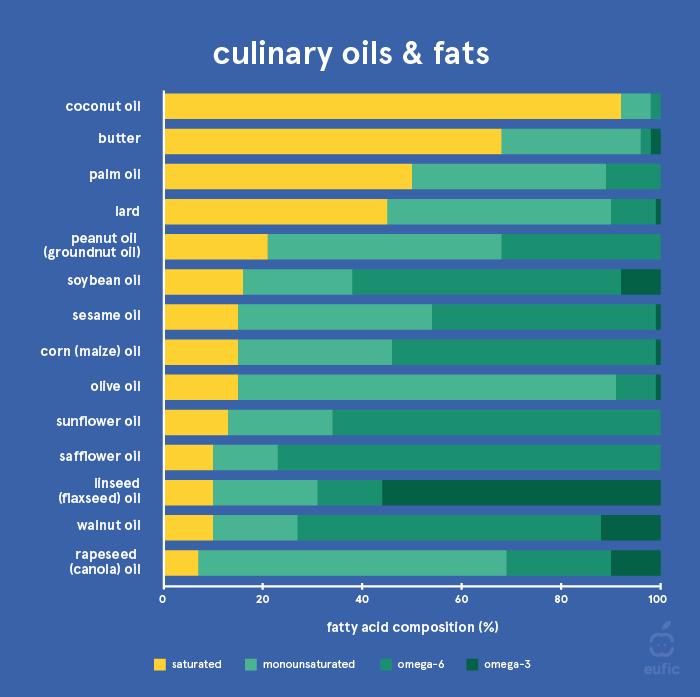
Olive oil is probably much better than other oils for normal frying for two reasons. Firstly it contains very low PUFAs, secondly it contains anti-oxidant compounds which might be protective against lipid peroxide formation.
Personally, I am not too worried about trans-fat formation in my own kitchen. The amount of formation is probably extremely low at anything resembling a normal cooking time. Unless you re-use cooking oils for days at a time, or cook at extreme temperatures, you’re most likely to be fine.
I am somewhat worried about the formation of lipid peroxides in my own cooking. I normally cook with olive oil or butter anyway, but in the rare case when I deep-fry things I’ll make two changes:
Try to use lower-PUFA oils for deep frying. Peanut/groundnut oil EDIT: Mustard oil might be even better will likely be my primary choice based on the intersection of PUFAs/price/ethics/flavour/availability.
Never re-use deep-frying oil
I think that peanut oil is less likely to cause health problems than other deep-frying oils, since it’s the main deep-frying oil in east Asia, which has much less obesity than the west, even in places like the Philippines where deep fried food is most common.
I am also somewhat concerned about trans-fat formation in commercial cooking, but this is dwarfed by the significant worries about oxidation from commercial cooking which will cash out as eating much fewer shop-bought deep-fried foods like crisps (chips) and chips (fries).
I think the PUFA breakdown hypothesis is more likely than the naive seed oil hypothesis, but I haven’t spent any time looking for counter-evidence yet.
As a quick point— McDonald’s fryers are not turned off as much as you think. At a 24 hour location, the fry/hash oil never turns off. The chicken fryer might be turned off between 4am and 11am if there’s no breakfast item containing chicken. Often it just gets left on so no one can forget to turn it on.
One thing to consider also, is the burnt food remaining in the fryers for many hours. Additionally, oil topped up between changes.
I don’t remember how often we changed the oil but I thought it was once per week. It was a 24 hour location
This seems like a huge civilizational inadequacy to me. This would be informative at large scale, and the cost would be very small.
It is also very easy to just do. Buy fries, extract fat in hexane, evaporate hexane and submit the fat you obtained for analysis.
Edit: It might even be possible to DIY the analysis if it is not commercially available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4609978/. (IR spectroscopy and AgNO3-DLC look somewhat accessible though I would have to look deeper into the topic to be sure.)
I’d be most interested in detecting hydroperoxides, which is easier than detecting trans fats. I don’t know how soluble a lipid hydroperoxide is in hexane, but isopropanol-hexane mixtures are often used for lipid extracts and would probably work better.
Evaporation could probably be done relatively safely by just leaving the extract at room temperature (I would definitely not advise heating the mixture at all) but you’d need good ventilation, preferably an outdoor space.
I think commercial LCMS/GCMS services are generally available to people in the USA/UK, and these would probably be the gold standard for detecting various hydroperoxides. I wouldn’t trust IR spectroscopy to distinguish the hydroperoxides from other OH-group containing contaminants when you’re working with a system as complicated as a box of french fries.
While I agree with the logic of avoiding subjecting highly unsaturated oils to heat we do have to be cautious here with speculation.
When you say things like that: “Nonetheless, if these things are poisonous at high concentrations, they’re probably not great at low concentrations.”
It does not clearly follow that such a dose-response exists. The word “hormesis” gets thrown around a lot in the lay press, and there is actually some truth there. Plenty of moderate (even genotoxic) stressors have health benefits at lower doses. Of course, I would not gorge on lipid hydroperoxide based on this, because we have better evidence-based “hormetic” stressors, but it also does not follow that lipid oxidation products at low doses are harmful.
False as worded. Not sure if this is because you’re oversimplifying a complex topic or were just unaware of some edge cases. E.g., vaccenic acid occurs in nature and is thought to be good for us, last I checked. There may be a few other natural species that are similarly harmless.
On the other hand, there are unnatural trans fats found in things like partially hydrogenated vegetable oils that are evidently bad enough for a government ban. If identical molecules are still getting in our food in significant amounts from other sources, that could be a problem.
Yeah i was unaware of vaccenic acid. I’ve edited the post to clarify.
It seems like you’re aware of this, but AFAICT surprisingly many people who speak out about seed oils aren’t, so it may be worth stating outright: uncooked or lightly cooked PUFAs are among the healthiest fats you can eat, come primarily from nuts and seeds (and fatty fish), and include all the essential and metabolically-important fatty acids. They diffuse faster across mitochondrial membranes for energy production, esterify and de-esterify at greater rates to provide energy between meals, and are preferentially stored in parts of the body where they’re less likely to cause health problems.
It’s been on my mind lately since I did a bit of a deep dive into fatty acid metabolism for my own weight loss efforts. I’ve always tended to carry all my weight in my midsection, and it’s pretty stubborn. So I’ve been upping unrefined/uncooked/gently cooked PUFAs, reducing saturated fats (except MCT-rich sources), intermittent fasting, timing carbs to mostly be eaten when I’m already relatively carb-depleted (so they’re not converted to saturated fats for storage), timing aerobic exercise to usually happen in a fasted state, and upping protein in general. So relatively more flax/chia/salmon/coconut/hemp hearts/whey protein, less dairy/beef. I don’t own a scale, but I lost 5 waist inches in less than 3 weeks without significantly reducing total calorie intake or increasing total calorie burn. Whether or not I’m losing much net weight, there’s definitely some recomposition and/or redistribution going on, and a couple of months in I have more (and more stable) physical and mental energy.
Many inflammatory pathways are tightly regulated, often because they involve chain reactions. Some of the PUFA metabolites, like 4HNE, can directly adduct to proteins and DNA. Considering we are systems meant to detect small amounts of damage in order to mount an inflammatory response, even the tiniest amount of some of those hydroperoxides can be problematic. Many of these molecules are reactive to UV light, and the most common report from the anti PUFA crowd is a complete stop to sunburns. Meaning people who used to burn within 10 minutes now require a few hours of direct exposure to get an inflammatory response. I can confirm this on myself (I live in the desert).
I wouldn’t just write off the naive anti-seed oil position either from a chemical point of view. Metabolism is absurdly complicated and finely tuned. Substituting a slightly different substrate into a poorly understood set of reactions and feedback loops is unlikely to go well.
There was very little linoleic acid in the diet we evolved to eat. Sure, it’s essential in small quantities, but using it as a major energy source is likely a very bad idea a priori.
One thing I like about the PUFA breakdown theory is that it agrees with aspects of so many different diets.
Keto avoids fried food because usually the food being fried is carbs
Carnivore avoids vegetable oils because they’re not meat
Paleo avoids vegetable oils because they weren’t available in the ancestral environment
Vegans tend to emphasize raw food and fried foods often have meat or cheese in them
Low-fat diets avoid fat of all kinds
Ray Peat was perhaps the closest to the mark in emphasizing that saturated fats are more stable (he probably talked about PUFA breakdown specifically, I’m not sure).
Edit: I originally wrote “neatly explains why so many different diets are reported to work”
I’ve also realized that it might explain the anomalous (i.e. after adjusting for confounders) effects of living at higher altitude. The lower the atmospheric pressure, the less oxygen available to oxidize the PUFAs. Of course some foods will be imported already full of oxidized FAs and that will be too late, but presumably a McDonalds deep fryer in Colorado Springs is producing less PUFAs/hour than a correspondingly-hot one in San Francisco.
This feels too crazy to put in the original post but it’s certainly interesting.
Measuring the composition of fryer oil at different times certainly seems like a good way to test both the original hypothesis and the effect of altitude.
Don’t forget the standard diet advice of avoiding “processed foods”. It’s unclear what exactly the boundary is, but I think “oil that has been cooking for weeks” probably counts.
Yeah, that boundary gets very confused very fast. I’ve come across articles, written by professionals for a general audience, calling whole wheat flour ultra-processed, and others listing cutting as a processing method that makes food less healthy. My general opinion of most standard diet advice is that it’s at about this level of reliability.
Really? I would only consider foods that were deliberately modified using procedures developed within the last century to be “processed”.
I think historically frying would have used olive oil or lard though.
I am confused by this sort of reasoning. As far as I’m aware, mainstream nutritional science/understanding already points towards avoiding refined oils (and refined sugars).
There’s already explainations for why cutting out refined oil is be beneficial.
There are already reasonable explainations for why all of those diets might be reported to work, at least in the short term.
I knew that those wise and good benefactors of humanity would turn out to have been warning us of the dangers of polyunsaturated fats all along.
They might want to mention it to people like my father, who, on the advice of his doctor, has been pretty much only eating polyunsaturated fats these last twenty years, for the good of his heart.
Or perhaps to McDonalds, who on the basis of a consumer-led campaign changed their famously good beef-dripping fried chips to vegetable-oil fried chips, coincidentally at about the time obesity and various other nasty diseases with no known cause really became fashionable in America.
I’m unsure exactly what points you’re making.
I’m saying the idea that it’s healthiest to avoid virtually any refined oil is mainstream nutritional understanding. Do you dispute this? I’m not making a point about which refined oils/fats are better than others. I haven’t seen anything that has convinced me mainstream nutrition is wrong about that, but I don’t think its particularly important when they can all be avoided.
Typical doctors are not particularly reliable nutritional authorities. They have almost no nutrition training.
MacDonalds fries are clearly very unhealthy regardless of what they’re fried in. Do you have evidence that they’re healthier when fried in beef tallow?
Regardless, the point I was making was that the diets the original commenter mentioned all restrict things that mainstream nutrition already suggests cause health problems.
Refined sugar, refined grains, refined fats, and animal products are all things mainstream nutrition suggests cause health problems. All of the diets listed restrict at least one of those things, so it’s not surprising that people would report temporary improvements in health relative to a diet that doesn’t restrict any of them.
You’re right, my original wording was too strong. I edited it to say that it agrees with so many diets instead of explains why they work.
See comment by Gilch, allegedly Vaccenic acid isn’t harmful. The particular trans-fats produced by isomerization of oleic and linoleic acid, however, probably are harmful. Elaidic acid for example is a major trans-fat component in margarines, which were banned.
Another thing linoleic acid does when there’s oxygen around is polymerize into a varnish, which is why linseed oil (lin-oleic) is traditionally used to waterproof cricket bats.
It used to say ‘do not eat’ in quite large letters on the cricket-bat-varnish bottles. Presumably now it says ‘heart-healthy!’.
In case you haven’t seen it, you might like dynomight’s recent post Thoughts on seed oil.
That post is part of what spurred this one